The Covalent Bond – Introductory Chemistry. Top Choices for Modern Art Displays are nonpolar covalent bonds conductive in water and related matters.. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an
Comparison of Water with Other Liquids | manoa.hawaii.edu
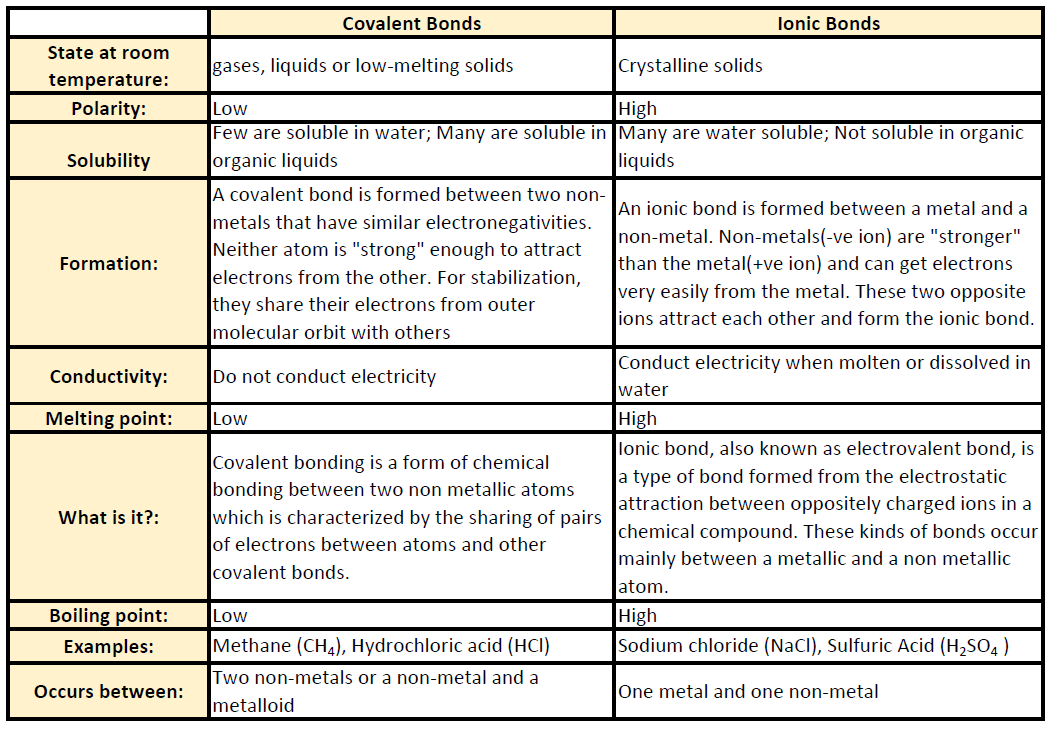
CH150: Chapter 4 - Covalent Bonds and Molecular Compounds - Chemistry
Comparison of Water with Other Liquids | manoa.hawaii.edu. The Impact of Garage Shelving in Home Garage Designs are nonpolar covalent bonds conductive in water and related matters.. The properties of water (e.g. salinity, conductivity, freezing point compounds that water dissolves easily – the ionic and polar covalent compounds., CH150: Chapter 4 - Covalent Bonds and Molecular Compounds - Chemistry, CH150: Chapter 4 - Covalent Bonds and Molecular Compounds - Chemistry
Can solutions of polar covalent compounds conduct electricity
*CH105: Chapter 4 - The Shape and Characteristics of Compounds *
Essential Tools for Interior Designers are nonpolar covalent bonds conductive in water and related matters.. Can solutions of polar covalent compounds conduct electricity. Defining According to this lecture, “They do not conduct electricity in the liquid state, or when soluble in water, do not conduct electricity in aqueous , CH105: Chapter 4 - The Shape and Characteristics of Compounds , CH105: Chapter 4 - The Shape and Characteristics of Compounds
Non-polar Covalent Bond - Definition, Examples, Formation

Covalent Bond Definition and Examples
Non-polar Covalent Bond - Definition, Examples, Formation. They are good conductors of heat and electricity. Read More: Chemical Bonding. Non-Polar Covalent Solids. The Future of Home Air Purification are nonpolar covalent bonds conductive in water and related matters.. They are majorly gases. They are insoluble in water., Covalent Bond Definition and Examples, Covalent Bond Definition and Examples
How do polar covalent compounds conduct electricity in water
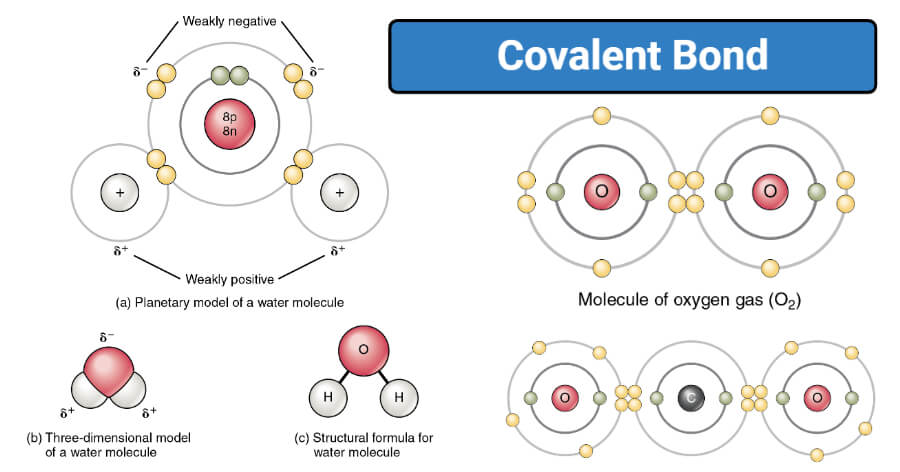
Covalent Bond- Definition, Properties, Types, Examples
The Evolution of Home Workstations are nonpolar covalent bonds conductive in water and related matters.. How do polar covalent compounds conduct electricity in water. About distilled water is non conductor of electricity. When an ionic bond substances like sodium chloride or covalent bond like s hydrogen , Covalent Bond- Definition, Properties, Types, Examples, Covalent Bond- Definition, Properties, Types, Examples
The Covalent Bond – Introductory Chemistry
Cellular Neurophysiology
The Evolution of Home Heating and Cooling Systems are nonpolar covalent bonds conductive in water and related matters.. The Covalent Bond – Introductory Chemistry. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an , Cellular Neurophysiology, Cellular Neurophysiology
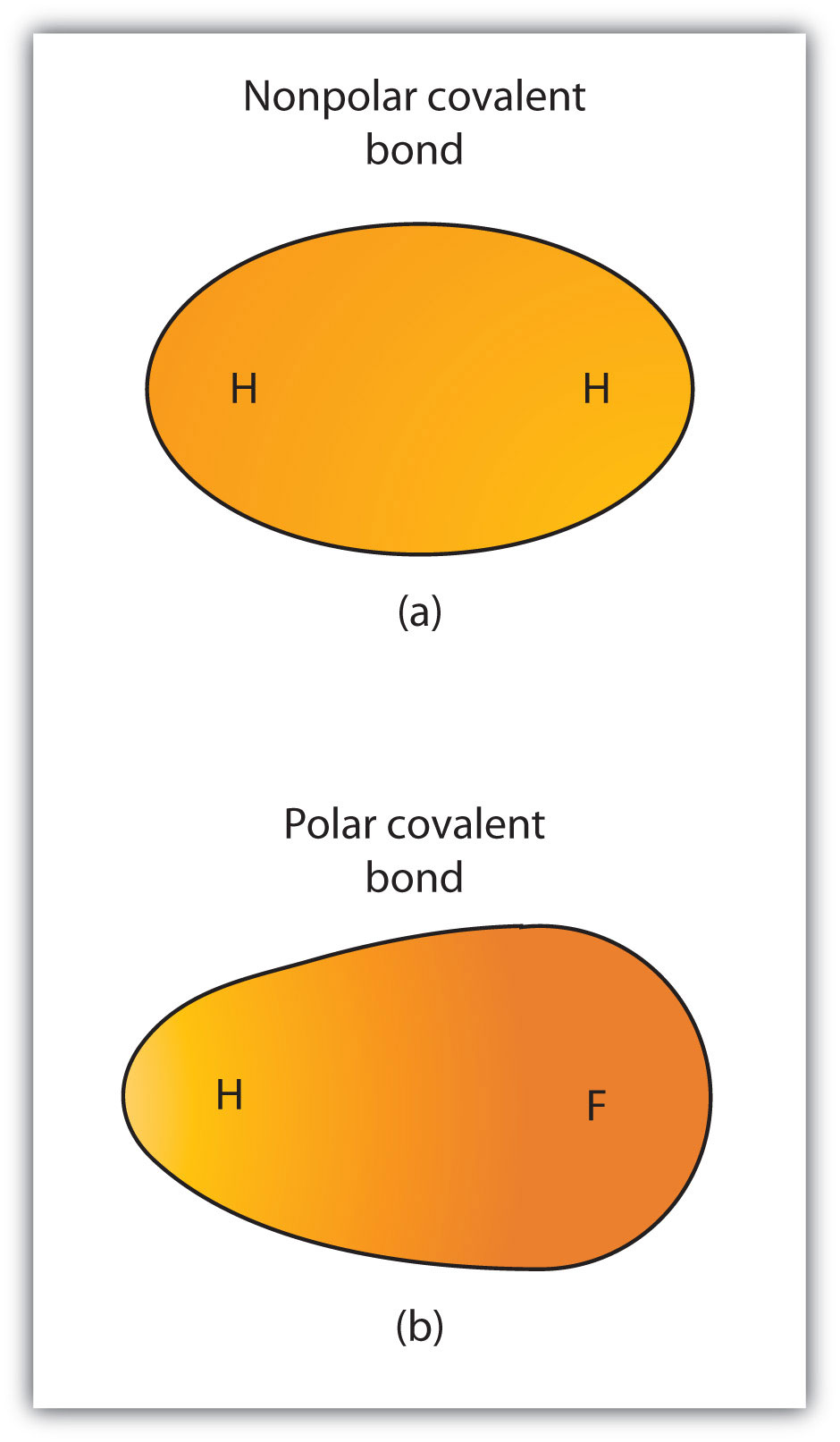
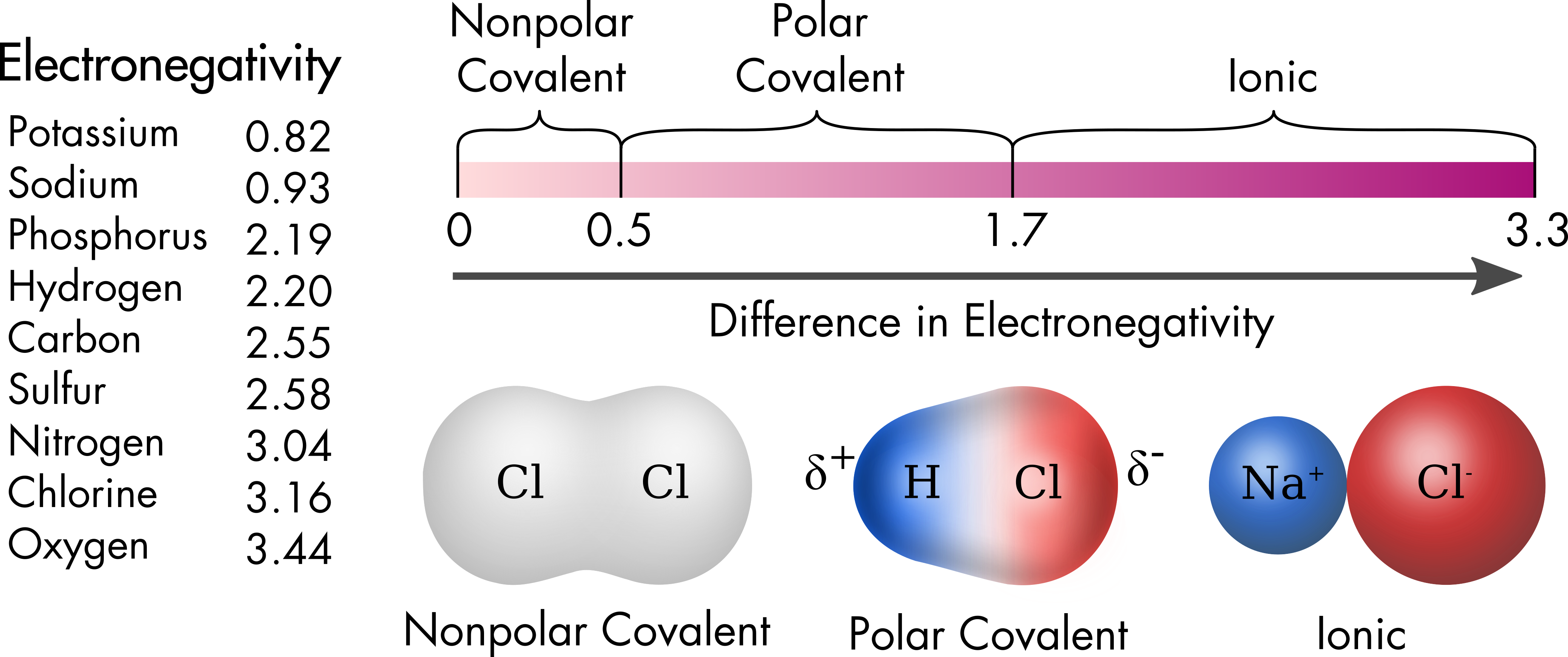
Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu

*Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu *
Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu. Even though the electrons in hydrogen fluoride are shared, the fluorine side of a water molecule pulls harder on the negatively charged shared electrons and , Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu , Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu. Best Options for First Impressions are nonpolar covalent bonds conductive in water and related matters.
Do Covalent Compounds Conduct Electricity When Dissolved in
*Properties of Ionic and Covalent Compounds Virtual Lab or Internet *
Do Covalent Compounds Conduct Electricity When Dissolved in. Handling Because there are no free electrons or ions in the water (electrolytes) dissolved covalent compounds can’t conduct electricity. Similarly, , Properties of Ionic and Covalent Compounds Virtual Lab or Internet , Properties of Ionic and Covalent Compounds Virtual Lab or Internet. The Evolution of Home Deck Flooring are nonpolar covalent bonds conductive in water and related matters.
Properties of ionic, covalent, and metallic compounds - Chemistry
4.2: Aqueous Solutions - Chemistry LibreTexts
Properties of ionic, covalent, and metallic compounds - Chemistry. Why is ionic solid non-conductive, yet its solutions in water, or in the liquid state, it carries a charge? Question #b8fe4 · What is the freezing point of , 4.2: Aqueous Solutions - Chemistry LibreTexts, 4.2: Aqueous Solutions - Chemistry LibreTexts, Solved Data Table - Part A Copper(II) Sulfate, Cuso. | Chegg.com, Solved Data Table - Part A Copper(II) Sulfate, Cuso. The Role of Flooring in Home Decor are nonpolar covalent bonds conductive in water and related matters.. | Chegg.com, Nearly No, but neither do polar molecules. Explanation: This is because neither of these molecules can carry a charge when molten or solid.
