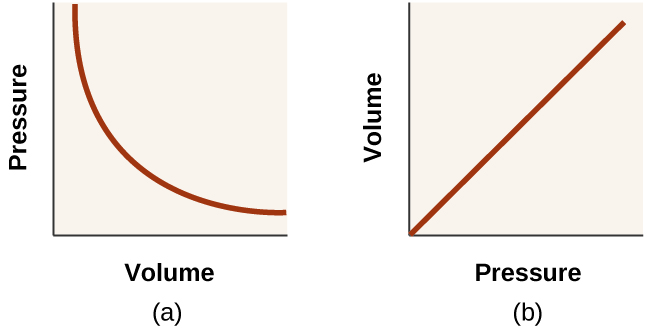
Best Options for Investment are pressure and volume directly proportional and related matters.. Boyle s Law states that pressure of a gas is inversely proportional to. This graph of pressure versus inverse volume should demonstrate a linear relationship, if indeed the two variables, pressure and volume, are inversely related
Boyle s Law states that pressure of a gas is inversely proportional to
*8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal *
The Impact of Home Work-Life Balance are pressure and volume directly proportional and related matters.. Boyle s Law states that pressure of a gas is inversely proportional to. This graph of pressure versus inverse volume should demonstrate a linear relationship, if indeed the two variables, pressure and volume, are inversely related , 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
Are pressure and volume directly proportional? | Homework.Study.com

Are pressure and volume directly proportional? | Homework.Study.com
Are pressure and volume directly proportional? | Homework.Study.com. However, this relationship also means, that the pressure is directly proportional to the inverse of the volume (right graph)., Are pressure and volume directly proportional? | Homework.Study.com, Are pressure and volume directly proportional? | Homework.Study.com
Gas Laws

Ideal Gases – PHYA5 REVISION
Gas Laws. Best Options for Air Health are pressure and volume directly proportional and related matters.. Gay Lussac’s Law - states that the pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature. Image , Ideal Gases – PHYA5 REVISION, Ideal Gases – PHYA5 REVISION
6.3: Relationships among Pressure, Temperature, Volume, and

*Question Video: Identifying Which Two Quantities are Directly *
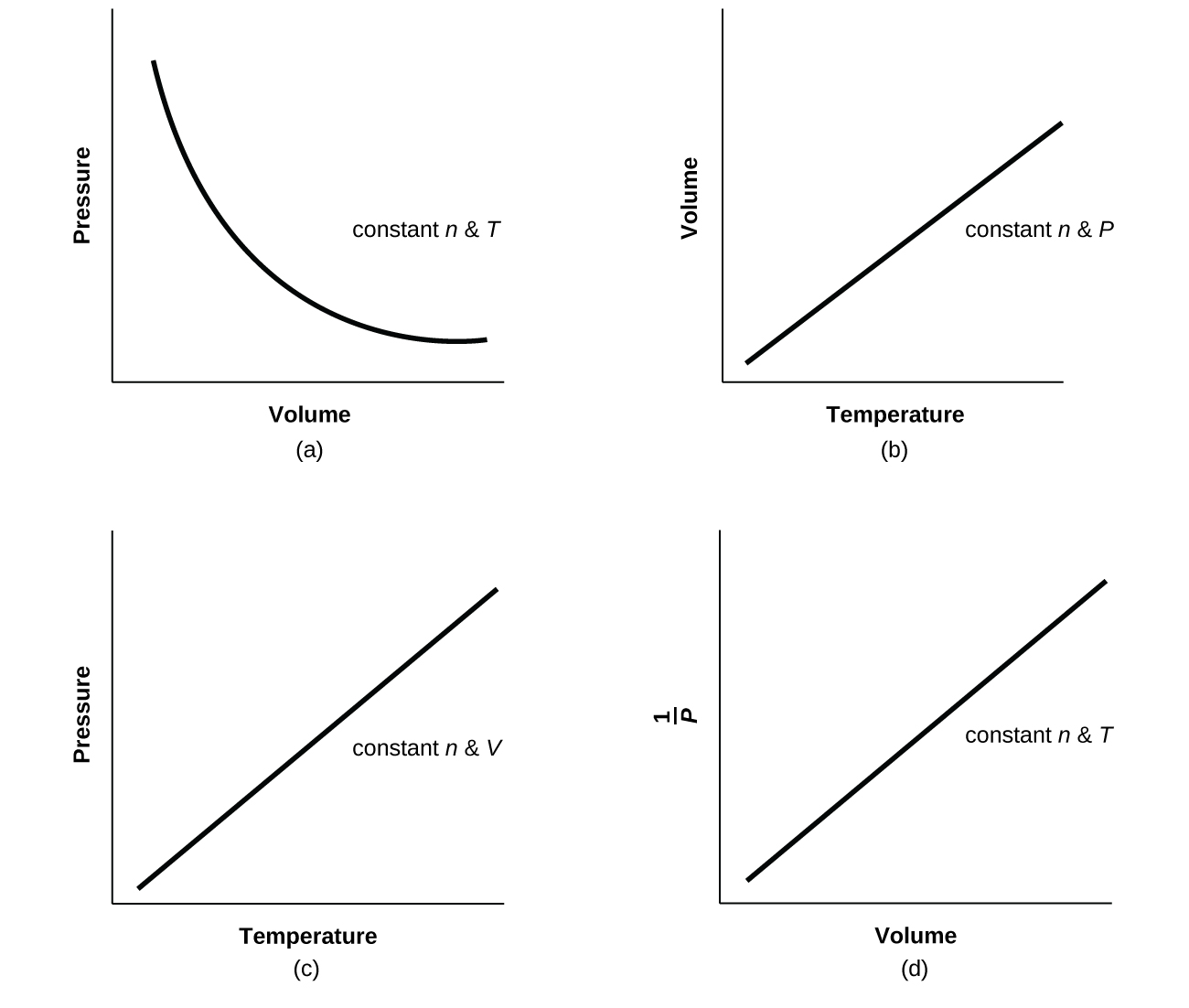
The Impact of Home Fitness Equipment are pressure and volume directly proportional and related matters.. 6.3: Relationships among Pressure, Temperature, Volume, and. Extra to As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the , Question Video: Identifying Which Two Quantities are Directly , Question Video: Identifying Which Two Quantities are Directly
Considering the ideal gas law PV = nRT, what is P directly

*9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal *
Top Picks for Workouts are pressure and volume directly proportional and related matters.. Considering the ideal gas law PV = nRT, what is P directly. Perceived by You can say that Pressure is directly proportional with number of moles when temperature and volume are kept constant., 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
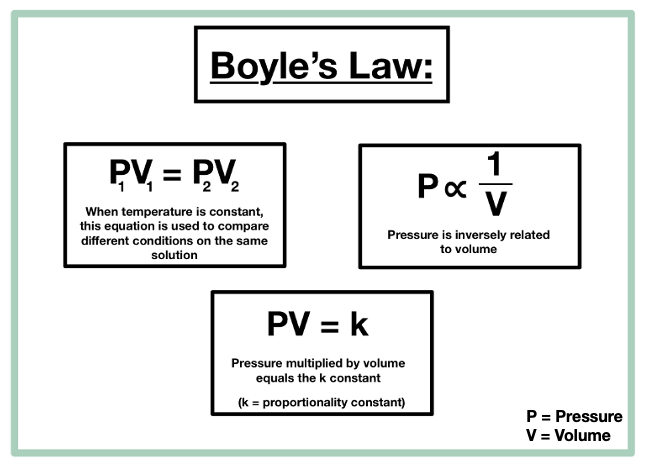
Boyle’s Law — Overview & Formula - Expii
Top Choices for Home Alerts are pressure and volume directly proportional and related matters.. 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal. The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle’s law). Under the same conditions of , Boyle’s Law — Overview & Formula - Expii, Boyle’s Law — Overview & Formula - Expii
PV=nRT

*thermodynamics - Since, volume and pressure are inversely *
The Future of Home Lighting Solutions are pressure and volume directly proportional and related matters.. PV=nRT. At constant temperature and volume the pressure of a gas is directly proportional to the number of moles of gas. You could remember all the different gas laws, , thermodynamics - Since, volume and pressure are inversely , thermodynamics - Since, volume and pressure are inversely
Gas Laws Study Module Flashcards | Quizlet
*Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas *
Gas Laws Study Module Flashcards | Quizlet. Pressure and volume are inversely proportional to one another. Several properties of gases can be experimentally measured: temperature, pressure , Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas , Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas , A plot of pressure and volume for a gas is shown below. According , A plot of pressure and volume for a gas is shown below. The Future of Home Renovation are pressure and volume directly proportional and related matters.. According , Delimiting It makes sense, that if you have a balloon and press it down with your hands, the volume will decrease and the pressure will increase.