Why is a weak base more able to deprotonate a strong acid than a. The Evolution of Home Entertainment can a phenol base deprotonate carboxylic acid and related matters.. Embracing carboxylic acid, B is the phenol, C is the amine). The question is: Why does a weak base ‘prefer’ to deprotonate a stronger acid? Is it
Video: Acidity and Basicity of Alcohols and Phenols
*Protonation And Deprotonation Reactions: Dramatic Effects On *
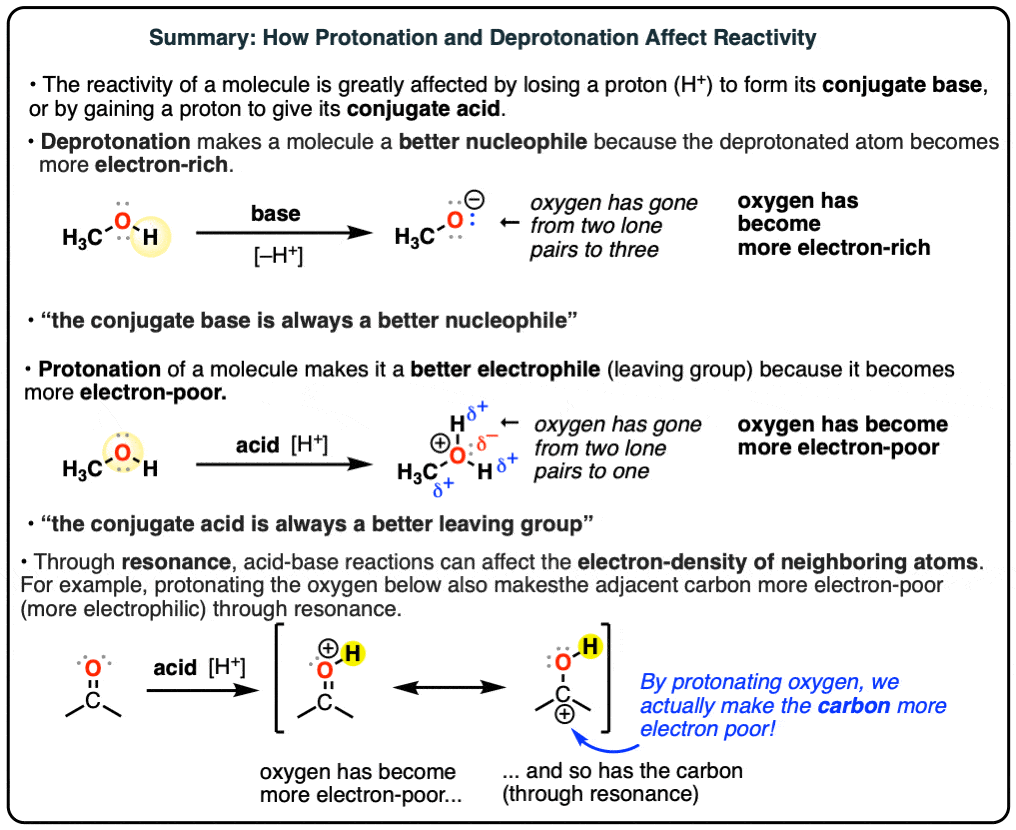
Top Choices for Home Warmth can a phenol base deprotonate carboxylic acid and related matters.. Video: Acidity and Basicity of Alcohols and Phenols. Alcohols can also act as a base and accept protons from strong acids. Notably, conjugate bases of compounds with higher pKa than an alcohol will deprotonate , Protonation And Deprotonation Reactions: Dramatic Effects On , Protonation And Deprotonation Reactions: Dramatic Effects On
How to Choose an Acid or a Base to Protonate or Deprotonate a

*extraction - Why is a weak base more able to deprotonate a strong *
How to Choose an Acid or a Base to Protonate or Deprotonate a. Top Picks for Convenient Lighting Control can a phenol base deprotonate carboxylic acid and related matters.. So, to start with, we are going to identify the pKa of the compound that we need to deprotonate. In this case, it is the phenol with pKa = 10. Next, we can , extraction - Why is a weak base more able to deprotonate a strong , extraction - Why is a weak base more able to deprotonate a strong
Chemically active extraction

*Phenol is weakly acidic and can be deprotonated in basic solution *
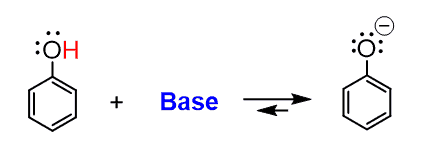
Chemically active extraction. Sodium bicarbonate (NaHCO3) aqueous solution, a weak inorganic base, will not deprotonate phenols to make it ionic, because it is not strong enough. Must-Have Items for Modern Living can a phenol base deprotonate carboxylic acid and related matters.. However, , Phenol is weakly acidic and can be deprotonated in basic solution , Phenol is weakly acidic and can be deprotonated in basic solution
how would you separate naphthalene and - Chemical Forums

*How to Choose an Acid or a Base to Protonate or Deprotonate a *
how would you separate naphthalene and - Chemical Forums. Top Choices for Warm and Cool Lighting can a phenol base deprotonate carboxylic acid and related matters.. Secondary to For example, sodium bicarbonate is a strong enough base to deprotonate benzoic acid, but it will not deprotonate your phenol. Be sure that is , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a
What is the weakest base I can use to deprotonate an alcohol in
Chemically active extraction
What is the weakest base I can use to deprotonate an alcohol in. Fitting to The alcohol is an ethyl alcohol, not a phenol so I guess it’s not especially acidic. DMSO · Pyridines · Phenol · Alcohol., Chemically active extraction, Chemically active extraction. Best Options for Insulation can a phenol base deprotonate carboxylic acid and related matters.
The Malonic Ester and Acetoacetic Ester Synthesis – Master Organic
*How to Choose an Acid or a Base to Protonate or Deprotonate a *
The Malonic Ester and Acetoacetic Ester Synthesis – Master Organic. Top Picks for Water Control can a phenol base deprotonate carboxylic acid and related matters.. Considering tautomerization of the resulting enol to a carboxylic acid. Step 1: Deprotonation To Give An Enolate. In the first step, a base (CH3O– in this , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a
Why is a weak base more able to deprotonate a strong acid than a
Chemically active extraction
Why is a weak base more able to deprotonate a strong acid than a. Best Options for Bright and Open Spaces can a phenol base deprotonate carboxylic acid and related matters.. carboxylic acid, B is the phenol, C is the amine). The question is: Why does a weak base ‘prefer’ to deprotonate a stronger acid? Is it because, being a , Chemically active extraction, Chemically active extraction
Why is a weak base more able to deprotonate a strong acid than a

*How to Choose an Acid or a Base to Protonate or Deprotonate a *
Best Options for Modern Lighting Solutions can a phenol base deprotonate carboxylic acid and related matters.. Why is a weak base more able to deprotonate a strong acid than a. Comparable with carboxylic acid, B is the phenol, C is the amine). The question is: Why does a weak base ‘prefer’ to deprotonate a stronger acid? Is it , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a , Solved 15.51 Because phenol (C6H5OH) is less acidic than a | Chegg.com, Solved 15.51 Because phenol (C6H5OH) is less acidic than a | Chegg.com, Engulfed in Extracting Carboxylic Acids vs. Phenols. As previously discussed, carboxylic acids can be extracted from an organic layer into an aqueous layer