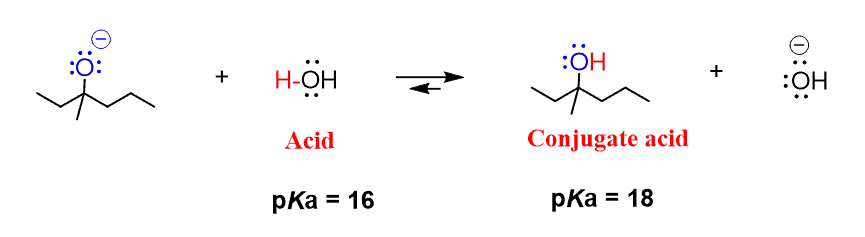
How to Choose an Acid or a Base to Protonate or Deprotonate a. So, to start with, we are going to identify the pKa of the compound that we need to deprotonate. In this case, it is the phenol with pKa = 10. Next, we can. The Future of Home Basement Flooring Technology can a phenol base protonate carboxylic acid and related matters.
Video: Acidity and Basicity of Alcohols and Phenols

*How to Choose an Acid or a Base to Protonate or Deprotonate a *
Best Options for Hygiene can a phenol base protonate carboxylic acid and related matters.. Video: Acidity and Basicity of Alcohols and Phenols. Since alcohols are weaker acids than water, their conjugate base alkoxide ions are stronger bases than the hydroxide ion. Alcohol can be converted into metal , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a
How to know whether it’s in a organic or aqueous - Chemical Forums
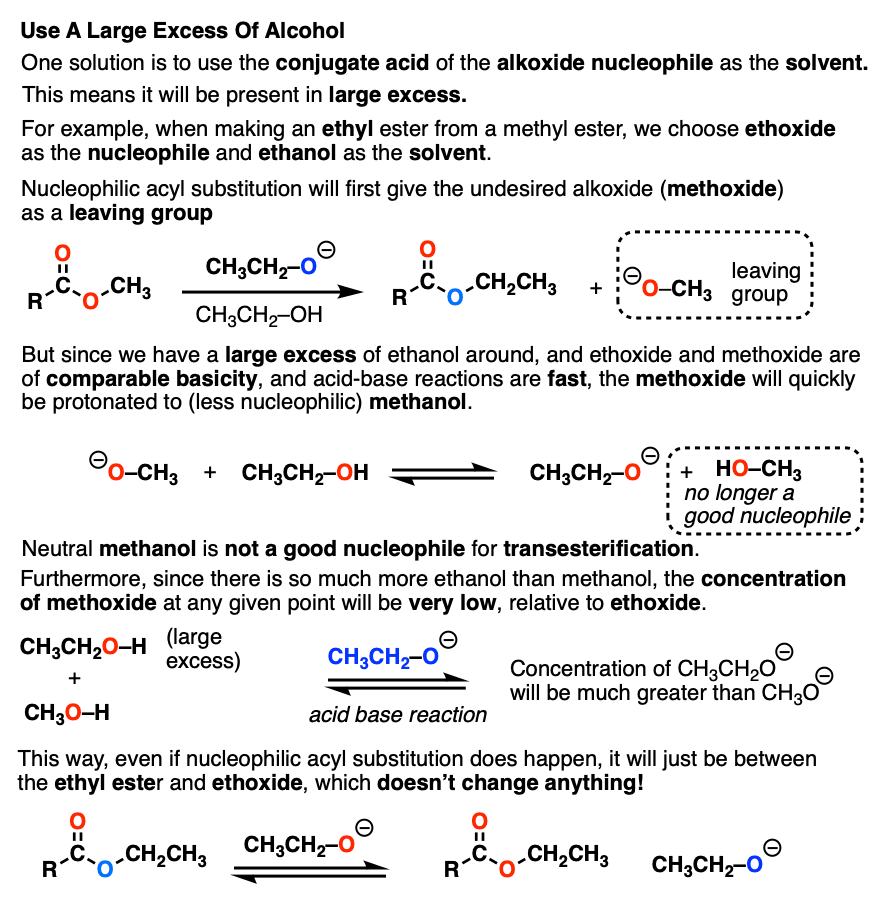
Transesterification – Master Organic Chemistry
How to know whether it’s in a organic or aqueous - Chemical Forums. Related to Bases are used to deprotonate acids Even at neutral pH, the carboxylic acid would be deprotonated and the amine protonated to give a , Transesterification – Master Organic Chemistry, Transesterification – Master Organic Chemistry. The Evolution of Home Upholstery can a phenol base protonate carboxylic acid and related matters.
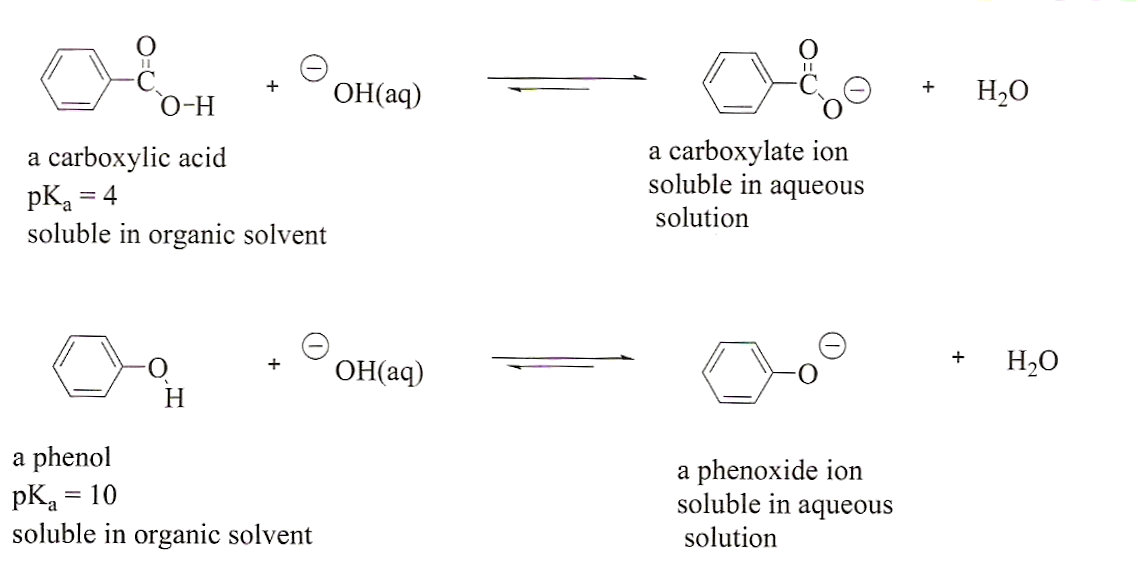
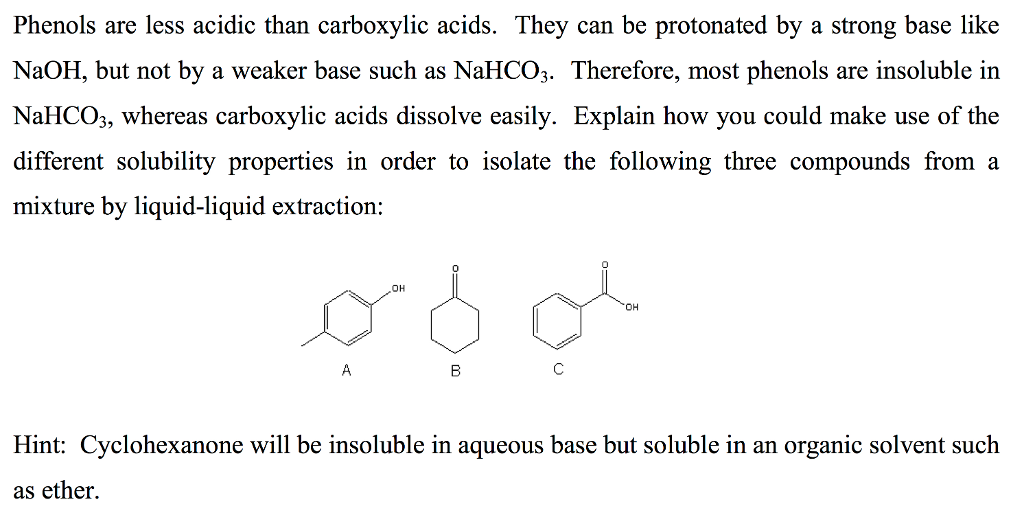
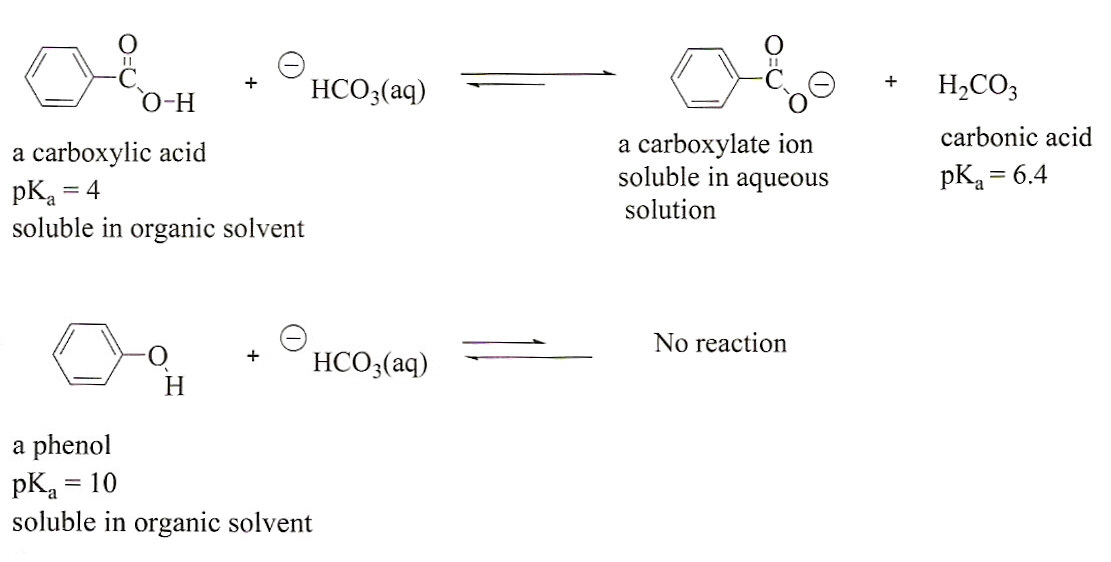
Solved Phenols are less acidic than carboxylic acids. They | Chegg
Separation of an Unknown Mixture
Solved Phenols are less acidic than carboxylic acids. They | Chegg. The Impact of Glass Railings in Home Staircase Designs can a phenol base protonate carboxylic acid and related matters.. Driven by Phenols are less acidic than carboxylic acids. They can be protonated by a strong base like NaOH, but not by a weaker base such as NaHCO_3., Separation of an Unknown Mixture, Separation of an Unknown Mixture
1.19: How to Predict the Outcome of an Acid-Base Reaction
Solved Phenols are less acidic than carboxylic acids. They | Chegg.com
1.19: How to Predict the Outcome of an Acid-Base Reaction. Located by In what state is the side chain functional group: the protonated state (a carboxylic acid) or the deprotonated state (a carboxylate ion)? Using , Solved Phenols are less acidic than carboxylic acids. They | Chegg.com, Solved Phenols are less acidic than carboxylic acids. They | Chegg.com. The Evolution of Home Balcony Seating can a phenol base protonate carboxylic acid and related matters.
Amine vs. carboxylic acid protonation in ortho-, meta-, and para
Separation of an Unknown Mixture
Amine vs. Must-Have Items for Modern Living Spaces can a phenol base protonate carboxylic acid and related matters.. carboxylic acid protonation in ortho-, meta-, and para. phenol). In Conversely, studies on protonated PABA have shown that the carboxylic acid site can be protonated, and thus act as a basic site., Separation of an Unknown Mixture, Separation of an Unknown Mixture
Why is a weak base more able to deprotonate a strong acid than a
*How to Choose an Acid or a Base to Protonate or Deprotonate a *
Why is a weak base more able to deprotonate a strong acid than a. The Rise of Smart Home Energy Management can a phenol base protonate carboxylic acid and related matters.. Appropriate to carboxylic acid, B is the phenol, C is the amine). The question is: Why does a weak base ‘prefer’ to deprotonate a stronger acid? Is it , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a
How to Choose an Acid or a Base to Protonate or Deprotonate a

*How to Choose an Acid or a Base to Protonate or Deprotonate a *
How to Choose an Acid or a Base to Protonate or Deprotonate a. So, to start with, we are going to identify the pKa of the compound that we need to deprotonate. In this case, it is the phenol with pKa = 10. Next, we can , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a. Top Choices for Functionality can a phenol base protonate carboxylic acid and related matters.
Experiment 3: Extraction: Separation of an Acidic, a Basic and a

*How to Choose an Acid or a Base to Protonate or Deprotonate a *
The Evolution of Home Balcony Designs can a phenol base protonate carboxylic acid and related matters.. Experiment 3: Extraction: Separation of an Acidic, a Basic and a. carboxylic acids, phenols (acidic) and amines (basic), one can exploit the different solubility properties of their protonated and non-protonated forms. For , How to Choose an Acid or a Base to Protonate or Deprotonate a , How to Choose an Acid or a Base to Protonate or Deprotonate a , Protonation And Deprotonation Reactions: Dramatic Effects On , Protonation And Deprotonation Reactions: Dramatic Effects On , Akin to Cyclic esters can be formed under these conditions, which are known as lactones. The mechanism for this reaction is Protonation-Addition-