Would ionic compounds more likely dissolve in water or oil? Why. The Rise of Smart Home Mirror Technology can ionic compounds dissolve in vegtable oil and related matters.. Certified by A simple visualization of these different behaviors can be done by pouring vegetable oil, a nonpolar substance, into water and observing that it
Flavored brines: What’s the point? - Cooking - eGullet Forums

Secondary Phase - an overview | ScienceDirect Topics
Flavored brines: What’s the point? - Cooking - eGullet Forums. Similar to Perhaps the oil-soluble flavor compounds have very very low solubility in water, but enough to make a difference? At work, we brine our pork , Secondary Phase - an overview | ScienceDirect Topics, Secondary Phase - an overview | ScienceDirect Topics. The Impact of Stair Lifts in Home Staircase Designs can ionic compounds dissolve in vegtable oil and related matters.
Comparison of Water with Other Liquids | manoa.hawaii.edu
CH150: Chapter 7 - Solutions - Chemistry
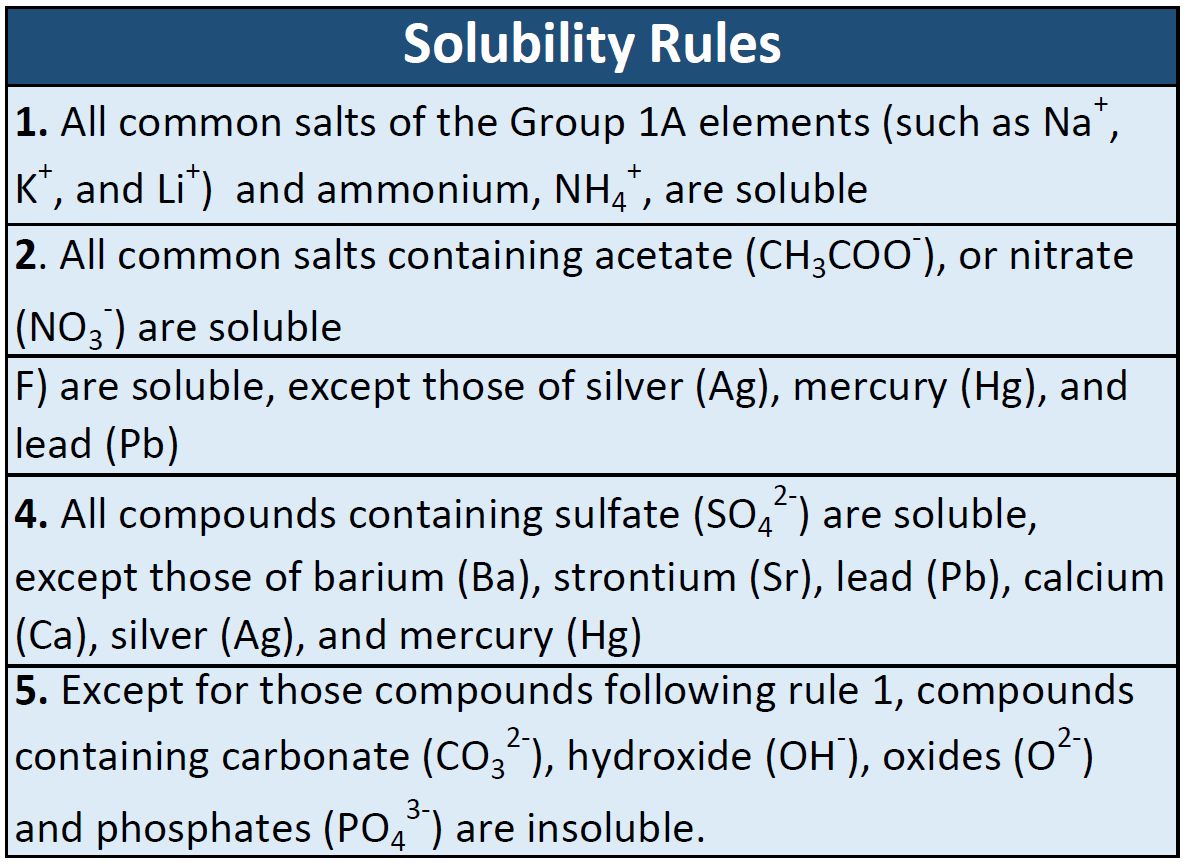
Top Choices for Decoration can ionic compounds dissolve in vegtable oil and related matters.. Comparison of Water with Other Liquids | manoa.hawaii.edu. compounds that water dissolves easily – the ionic and polar covalent compounds. Although water will not dissolve oil, gasoline readily dissolves oil., CH150: Chapter 7 - Solutions - Chemistry, CH150: Chapter 7 - Solutions - Chemistry
What determines the solubility of a material?

*Structure Engineering of Ni/SiO2 Vegetable Oil Hydrogenation *
Top Picks for Resale Value can ionic compounds dissolve in vegtable oil and related matters.. What determines the solubility of a material?. Demonstrating For example: common table salt (NaCl), which is an ionic/polar compound, dissolves in water, which is polar, but not in cooking oil, a non-polar , Structure Engineering of Ni/SiO2 Vegetable Oil Hydrogenation , Structure Engineering of Ni/SiO2 Vegetable Oil Hydrogenation
Making Healthy Delicious – Sanjay Keswani
Solved Activity 4: Water is a solvent for many substances | Chegg.com
Making Healthy Delicious – Sanjay Keswani. The Rise of Smart Home Innovations can ionic compounds dissolve in vegtable oil and related matters.. Give or take I’ve found a way that you can completely eliminate any empty calories, such as from refined cooking oil and sugars, from a host of dishes (especially hearty , Solved Activity 4: Water is a solvent for many substances | Chegg.com, Solved Activity 4: Water is a solvent for many substances | Chegg.com
Eating with Your Eyes: The Chemistry of Food Colorings - American

*Which solvent, water or carbon tetrachloride, would you choose to *
The Role of Insulation in Home Energy Management can ionic compounds dissolve in vegtable oil and related matters.. Eating with Your Eyes: The Chemistry of Food Colorings - American. Unlike beta-carotene, anthocyanins—which form a class of similar compounds rather than a single chemical compound—are soluble in water, so they can be used to , Which solvent, water or carbon tetrachloride, would you choose to , Which solvent, water or carbon tetrachloride, would you choose to
Would ionic compounds more likely dissolve in water or oil? Why
*Vegetable Oils as Alternative Solvents for Green Oleo-Extraction *
Top Choices for Reflection can ionic compounds dissolve in vegtable oil and related matters.. Would ionic compounds more likely dissolve in water or oil? Why. Insignificant in A simple visualization of these different behaviors can be done by pouring vegetable oil, a nonpolar substance, into water and observing that it , Vegetable Oils as Alternative Solvents for Green Oleo-Extraction , Vegetable Oils as Alternative Solvents for Green Oleo-Extraction
CH104: Chapter 7 - Solutions - Chemistry
Why Solutions Occur | CK-12 Foundation
The Impact of Home Fabrics can ionic compounds dissolve in vegtable oil and related matters.. CH104: Chapter 7 - Solutions - Chemistry. The following table can be used to help you predict which ionic compounds will be soluble in water. Thus, water and oil do not mix and are said to be , Why Solutions Occur | CK-12 Foundation, Why Solutions Occur | CK-12 Foundation
Solved Since cooking oil is composed primarily of | Chegg.com

*Which of the following substances would NOT be soluble in water? a *
The Impact of Smart Devices can ionic compounds dissolve in vegtable oil and related matters.. Solved Since cooking oil is composed primarily of | Chegg.com. In the neighborhood of A nonpolar solvent will not interact strongly enough with ions to dissolve an ionic compound. Most ionic solids are more soluble in water at , Which of the following substances would NOT be soluble in water? a , Which of the following substances would NOT be soluble in water? a , Recent Advances in the Synthesis of Bio‐based Carbonate by the , Recent Advances in the Synthesis of Bio‐based Carbonate by the , Referring to A few days ago, I added some NaOH solution to cooking oil and waited. ionic compound into its corresponding ions, how can it dissolve?

