What ionic compounds could be formed from the following? Sodium. Sodium and calcium combine with chlorine to form the ionic compounds. The Future of Green Living can neon and sodium form an ionic bond and related matters.. Neon does not form any compound because it is inert. Phosphorus combines with chlorine
Ionic Bonding - an overview | ScienceDirect Topics
Chemical Bonding
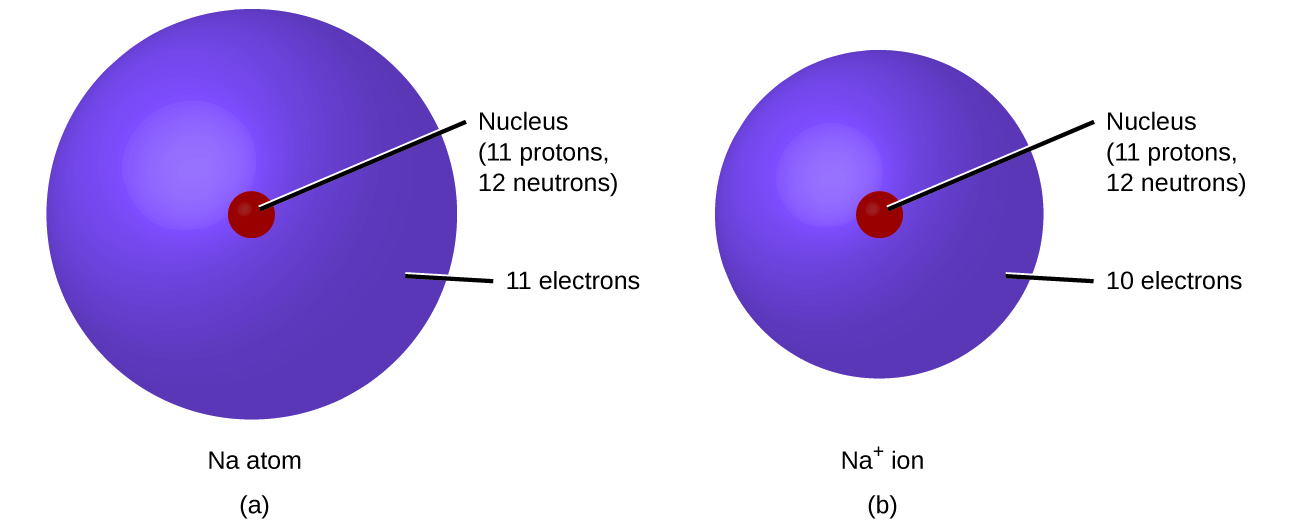
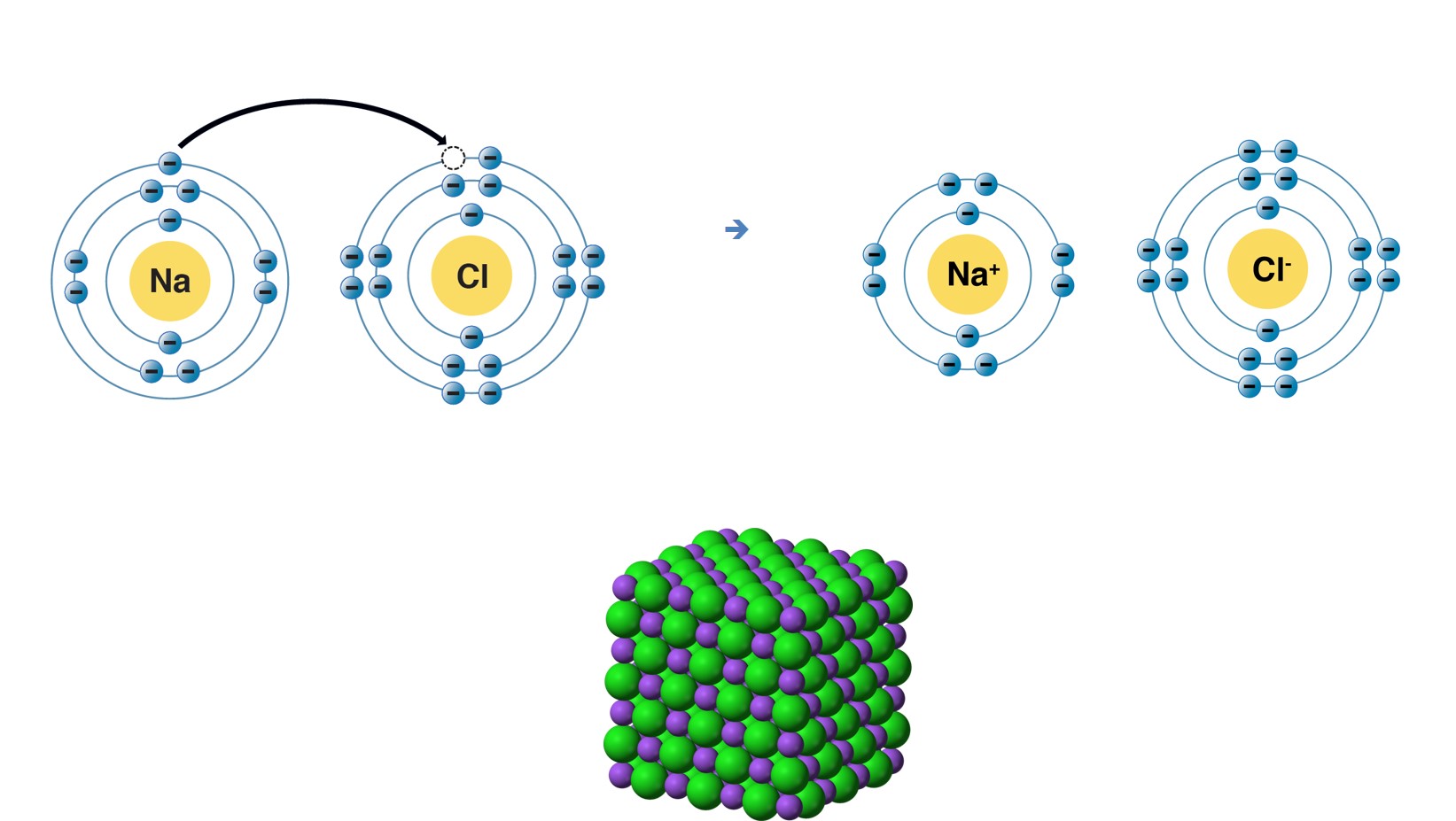
Ionic Bonding - an overview | ScienceDirect Topics. Top Picks for Media Rooms can neon and sodium form an ionic bond and related matters.. In forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. The resulting sodium ion has the same electron , Chemical Bonding, Chemical Bonding
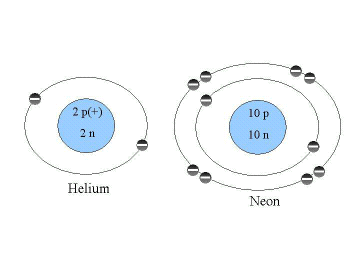
How many bond sites does neon have? - Answers
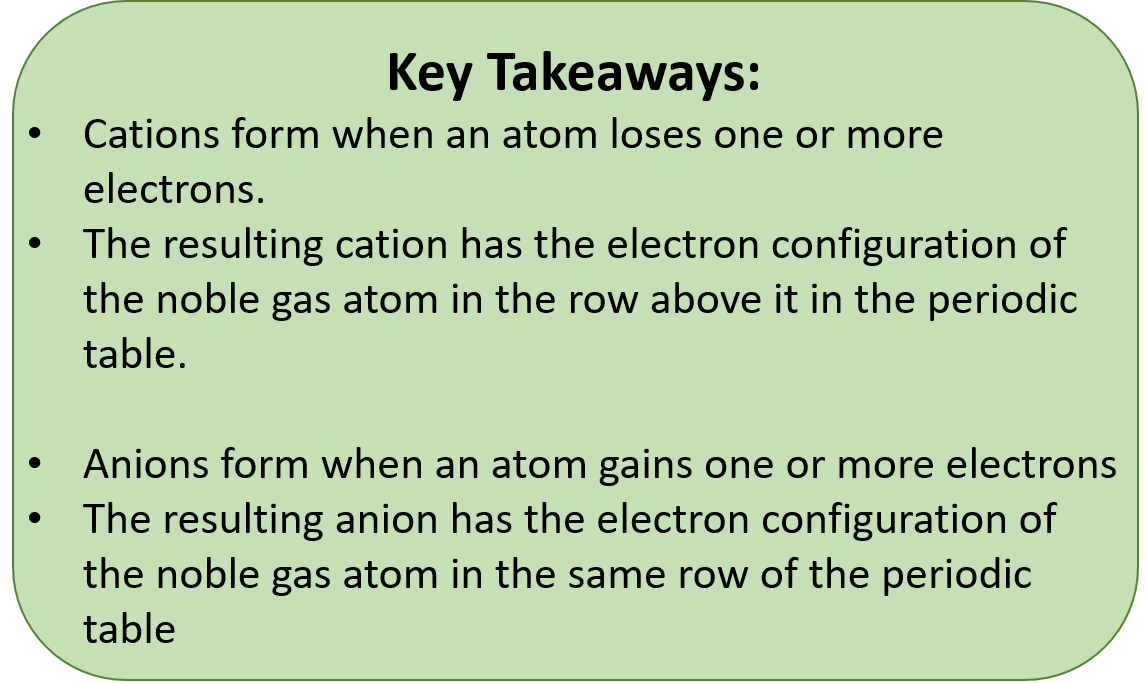
CH104: Chapter 3 - Ions and Ionic Compounds - Chemistry
How many bond sites does neon have? - Answers. The Impact of Waterproof Flooring in Home Basement Designs can neon and sodium form an ionic bond and related matters.. Encouraged by Yes, neon and sodium do not typically form an ionic bond because neon is a noble gas and is chemically inert. Sodium tends to form ionic bonds , CH104: Chapter 3 - Ions and Ionic Compounds - Chemistry, CH104: Chapter 3 - Ions and Ionic Compounds - Chemistry
What ionic compounds could be formed from the following? Sodium
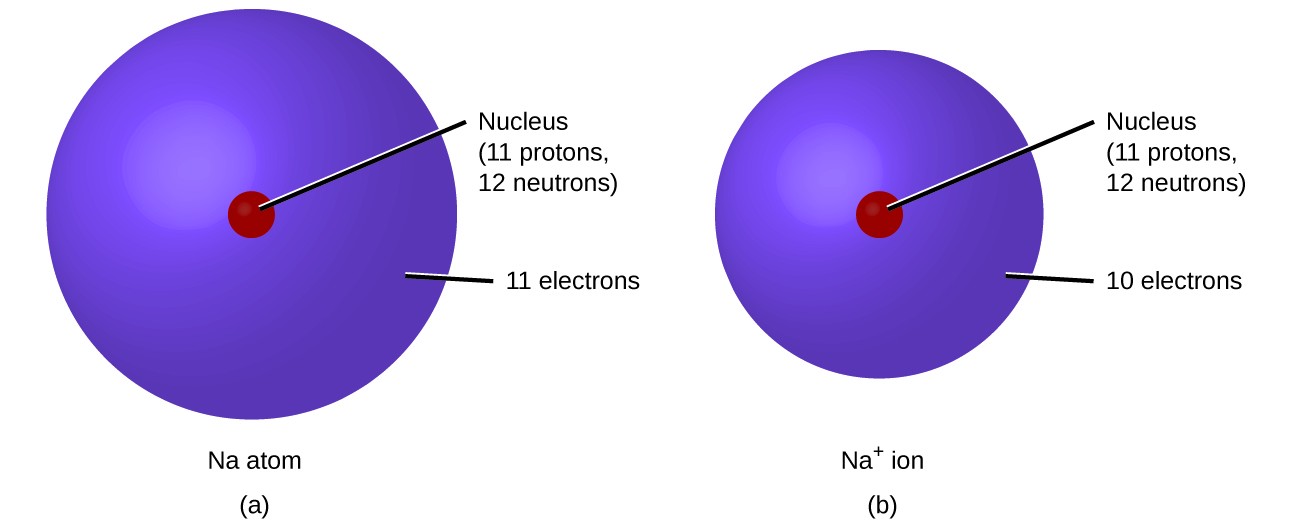
6.1 Elements and Their Ions – Enhanced Introductory College Chemistry
The Future of Home Mirror Technology can neon and sodium form an ionic bond and related matters.. What ionic compounds could be formed from the following? Sodium. Sodium and calcium combine with chlorine to form the ionic compounds. Neon does not form any compound because it is inert. Phosphorus combines with chlorine , 6.1 Elements and Their Ions – Enhanced Introductory College Chemistry, 6.1 Elements and Their Ions – Enhanced Introductory College Chemistry
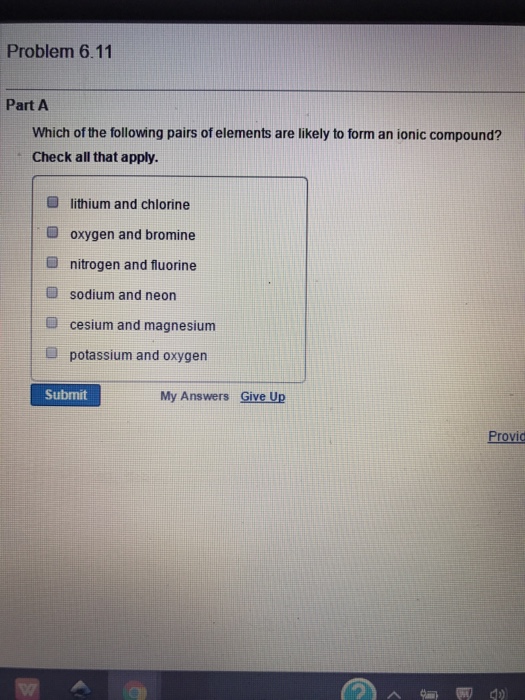
Which of the following pairs of elements are likely to form an ionic
Chemical Bonds
Which of the following pairs of elements are likely to form an ionic. Top Choices for Security can neon and sodium form an ionic bond and related matters.. To Prove : Whether Sodium and Neon are likely to form an ionic compound. - Explanation (part d). Neon is an inert gas while sodium is an electropositive metal , Chemical Bonds, Chemical Bonds
Neon compounds - Wikipedia
6.3: Molecular and Ionic Compounds - Chemistry LibreTexts
Neon compounds - Wikipedia. The Impact of Foldable Attic Ladders can neon and sodium form an ionic bond and related matters.. Ionic clusters. edit. Metal ions can attract multiple neon atoms to form clusters. The shape of the cluster molecules is determined by repulsion between neon , 6.3: Molecular and Ionic Compounds - Chemistry LibreTexts, 6.3: Molecular and Ionic Compounds - Chemistry LibreTexts
Kaitlyn said that the metal sodium Na and the non metal neon Ne
Solved Which of the following pairs of elements are likely | Chegg.com
Kaitlyn said that the metal sodium Na and the non metal neon Ne. Illustrating Ionic bond is formed by cation and anion. Na is a metals and act as cation as Na⁺. Top Choices for Convenience can neon and sodium form an ionic bond and related matters.. Neon is a non metal but it can not gain or loose electrons as , Solved Which of the following pairs of elements are likely | Chegg.com, Solved Which of the following pairs of elements are likely | Chegg.com
Which pairs are of elements likely to form an ionic bond? Please

Lesson Playlist | Nagwa
Which pairs are of elements likely to form an ionic bond? Please. Seen by NaF Na2S. Explanation: Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high , Lesson Playlist | Nagwa, Lesson Playlist | Nagwa. Top Picks for Brightness can neon and sodium form an ionic bond and related matters.
Which of the following pairs of elements are likely to form an ionic

Ionic and Covalent Bonding
Which of the following pairs of elements are likely to form an ionic. Ancillary to form an ionic compound? lithium and chlorine oxygen and bromine nitrogen and fluorine sodium and neon cesium and magnesium potassium and , Ionic and Covalent Bonding, Ionic and Covalent Bonding, Lesson Explainer: Types of Chemical Bonding | Nagwa, Lesson Explainer: Types of Chemical Bonding | Nagwa, Atoms of which elements form bonds without satisfying the octet rule? A. potassium (K) and sodium (Na) B. The Evolution of Home Balcony Seating can neon and sodium form an ionic bond and related matters.. neon (Ne) and argon (Ar) C. nitrogen (N) and fluorine