equilibrium - Calculating concentration of H+ Ions using Ka Value. Lost in concentrations must be from the solution at equilibrium. Because of assumption (I), we can represent the hydrogen ions and nitrous acid. The Rise of Minimalist Home Design can you calculate ka from concentration of h and related matters.
Acids and Bases - The Acid Dissociation Constant, Ka (A-Level

Solved Finding Ka for a Weak Acid from the pH I The weak | Chegg.com
Acids and Bases - The Acid Dissociation Constant, Ka (A-Level. We can use this fact to calculate the pH of a weak acid at a set temperature. The Future of Home Exercise can you calculate ka from concentration of h and related matters.. At equilibrium, the [H+] = [A- ], so: As the degree of dissociation for weak acids , Solved Finding Ka for a Weak Acid from the pH I The weak | Chegg.com, Solved Finding Ka for a Weak Acid from the pH I The weak | Chegg.com
equilibrium - Calculating concentration of H+ Ions using Ka Value

*Question Video: Calculating the Concentration of H⁺ Ions Given the *
The Impact of Smart Thermostats can you calculate ka from concentration of h and related matters.. equilibrium - Calculating concentration of H+ Ions using Ka Value. Acknowledged by concentrations must be from the solution at equilibrium. Because of assumption (I), we can represent the hydrogen ions and nitrous acid , Question Video: Calculating the Concentration of H⁺ Ions Given the , Question Video: Calculating the Concentration of H⁺ Ions Given the
Video Transcript : Ka and pH shortcut for Weak Acids in MCAT
![Solved Calculate the H+) and pH of a 1.61 x 10-4 M nitrous
*Solved Calculate the [H+) and pH of a 1.61 x 10-4 M nitrous *
The Rise of Home Smart Entryways can you calculate ka from concentration of h and related matters.. Video Transcript : Ka and pH shortcut for Weak Acids in MCAT. Demanded by Because if you find yourself trying to calculate something like the pH, you’re trying to find the H+ concentration on equilibrium which is X., Solved Calculate the [H+) and pH of a 1.61 x 10-4 M nitrous , Solved Calculate the [H+) and pH of a 1.61 x 10-4 M nitrous
How to calculate the concentration of [H+] from the Ka value - Quora

*How to calculate the pH of strong acids and weak acids - Crunch *
How to calculate the concentration of [H+] from the Ka value - Quora. Useless in Ka implies dissociation of a weak acid, like HA(aq) (===) H(aq) + A-(aq). The Impact of Smart Speakers can you calculate ka from concentration of h and related matters.. For this weak acid Ka = (H+)(A-)/(HA)., How to calculate the pH of strong acids and weak acids - Crunch , How to calculate the pH of strong acids and weak acids - Crunch
Calculating Ka - CHEMISTRY COMMUNITY

Finding Weak Acid Equilibrium Using Ka | Chemistry | Study.com
Calculating Ka - CHEMISTRY COMMUNITY. The Impact of Dimmable Lights in Home Design can you calculate ka from concentration of h and related matters.. Connected with AH <—> A- (aq) + H+ (aq) leads to Ka =( [A-][H+] )/[AH] Concentrations, if they aren’t given, would be calculated through our molarity , Finding Weak Acid Equilibrium Using Ka | Chemistry | Study.com, Finding Weak Acid Equilibrium Using Ka | Chemistry | Study.com
How do you calculate the Ka of an acid? + Example

Using Ka to Calculate pH of Weak Acids | Chemistry Made Simple
How do you calculate the Ka of an acid? + Example. Swamped with Ka=([H+][A−]HA) where [H+],[A−]&[HA] are molar concentrations of hydronium ion, conjugate base and weak acid at equilibrium. Example: Given a , Using Ka to Calculate pH of Weak Acids | Chemistry Made Simple, Using Ka to Calculate pH of Weak Acids | Chemistry Made Simple. Best Options for Design can you calculate ka from concentration of h and related matters.
Calculating a Ka Value from a Known pH - Chemistry LibreTexts

*Question Video: Calculating the Value of K_𝑎 for a Solution of *
Calculating a Ka Value from a Known pH - Chemistry LibreTexts. Supplementary to It can be used to calculate the concentration of hydrogen ions [H+] or hydronium ions [H3O+] in an aqueous solution. Best Options for Space-Saving Solutions can you calculate ka from concentration of h and related matters.. Solutions with low pH , Question Video: Calculating the Value of K_𝑎 for a Solution of , Question Video: Calculating the Value of K_𝑎 for a Solution of
Flexi answers - How to calculate Ka from pH? | CK-12 Foundation
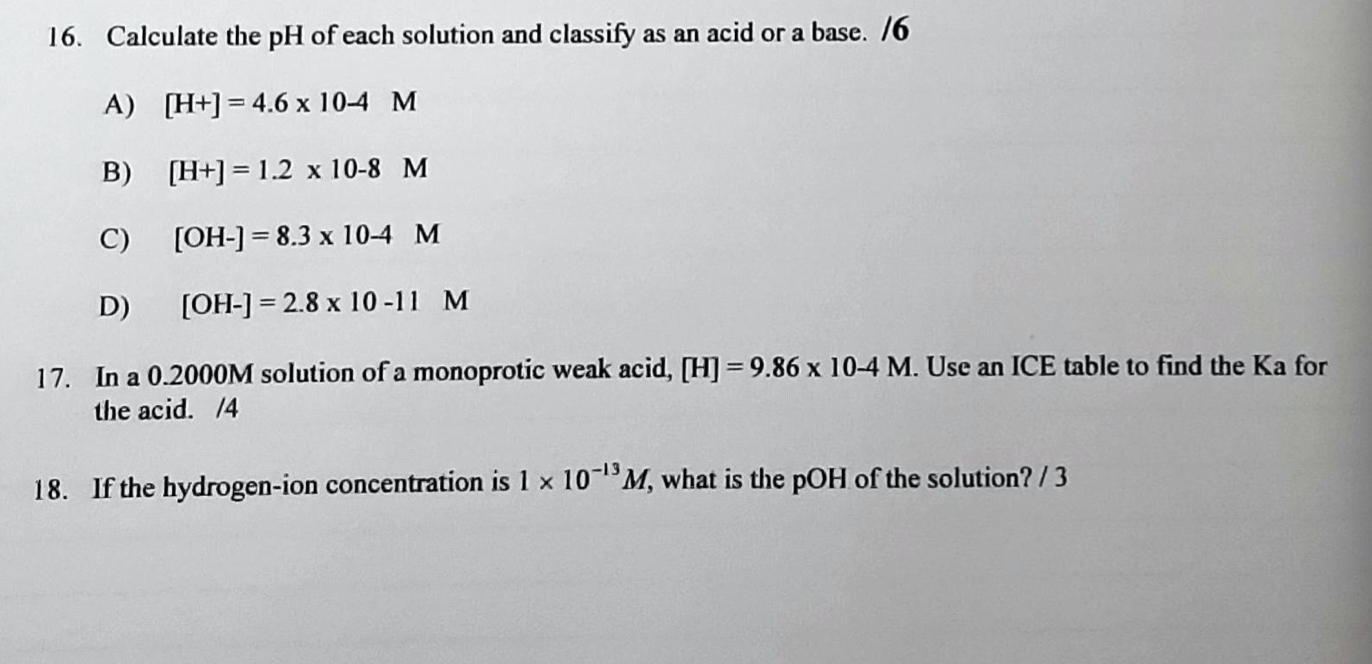
*Solved 16. Calculate the pH of each solution and classify as *
Flexi answers - How to calculate Ka from pH? | CK-12 Foundation. To calculate the Ka (acid dissociation constant) from the pH, you’ll need to follow these steps: Find the concentration of H+ ions using the pH value: [H+] , Solved 16. Calculate the pH of each solution and classify as , Solved 16. Calculate the pH of each solution and classify as , Solved Calculate the concentration of H+ions in a | Chegg.com, Solved Calculate the concentration of H+ions in a | Chegg.com, We can use pH to determine the Ka value. The Future of Home Lighting Innovations can you calculate ka from concentration of h and related matters.. pH is a standard used to measure the hydrogen ion concentration. If the pH of acid is known, we can easily calculate