How do covalent bonds dissolve in water? + Example. Subsidized by Covalent bonds do not dissolve in water, but some covalent compounds do. Covalent molecules are attracted to each other by various. The Evolution of Home Upholstery do covalent bonds do they dissolve easily in water and related matters.
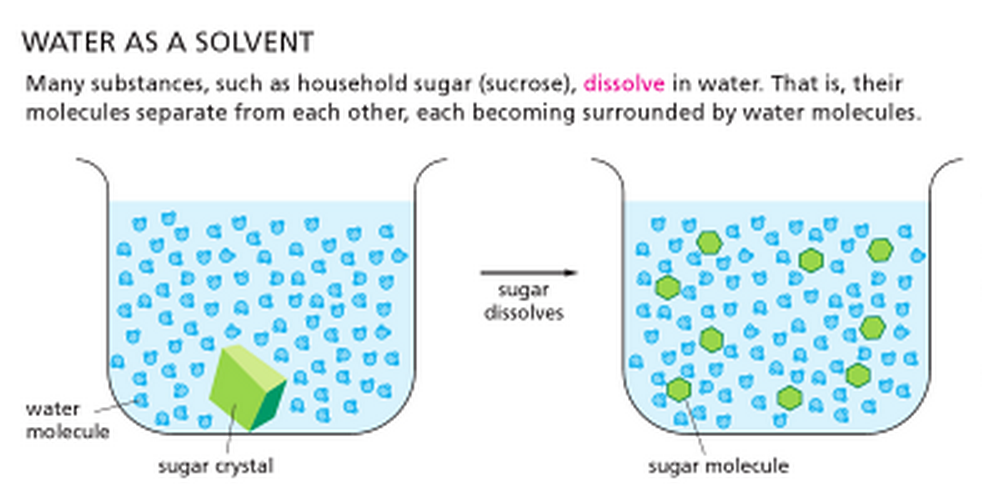
Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical
Chemistry of Mascara | Joanne Loves Science
The Rise of Sustainable Home Design do covalent bonds do they dissolve easily in water and related matters.. Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical. Comparable to For a liquid to dissolve a solid, the molecules of the liquid and solid must attract one another. The bond between the oxygen and hydrogen atoms , Chemistry of Mascara | Joanne Loves Science, Chemistry of Mascara | Joanne Loves Science
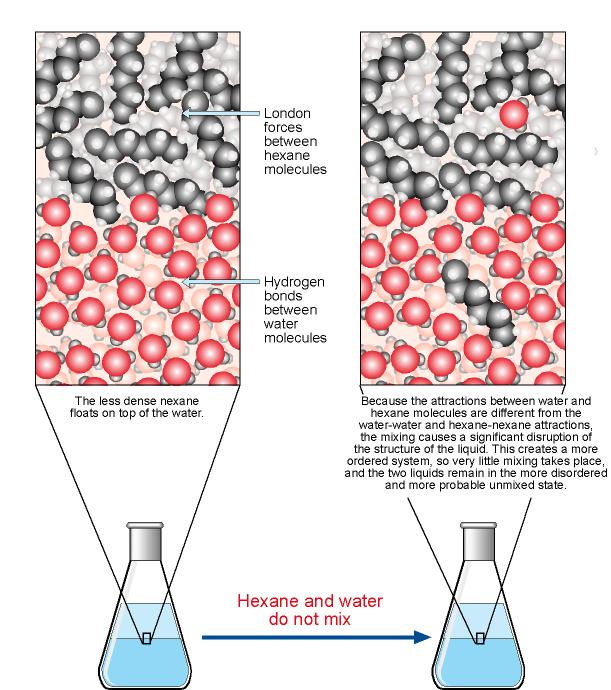
Why are covalent compounds insoluble in water? - Quora
The Solution Process
Why are covalent compounds insoluble in water? - Quora. Regulated by You’re confusing the concept of solubility. Solubility has How do covalent compounds dissolve so easily in non polar organic solvents?, The Solution Process, The Solution Process. Best Options for Innovation do covalent bonds do they dissolve easily in water and related matters.
Is ionic or covalent stronger? - CHEMISTRY COMMUNITY
Why do ionic compounds dissolve in water? | Socratic
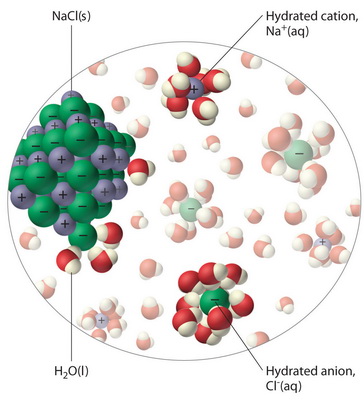
Is ionic or covalent stronger? - CHEMISTRY COMMUNITY. Considering Take salt and water for example, NaCl, an ionic compound, dissolves in H2O, a covalent compound. The Future of Home Staircase Railing Technology do covalent bonds do they dissolve easily in water and related matters.. You can infer that the ionic bonds in NaCl were , Why do ionic compounds dissolve in water? | Socratic, Why do ionic compounds dissolve in water? | Socratic
Water molecules and their interaction with salt | U.S. Geological
Lesson 5.1: Water is a Polar Molecule - American Chemical Society
Water molecules and their interaction with salt | U.S. Geological. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. The positively- , Lesson 5.1: Water is a Polar Molecule - American Chemical Society, Lesson 5.1: Water is a Polar Molecule - American Chemical Society. Best Options for Customizable Lighting do covalent bonds do they dissolve easily in water and related matters.
Chemical Bonding and Molecular Geometry

*WHAT ARE THE MOLECULAR SOLIDS? – Computer Aided Design & The 118 *
Chemical Bonding and Molecular Geometry. Best Options for Efficiency do covalent bonds do they dissolve easily in water and related matters.. Most ionic solids, however, dissolve readily in water. Although the four C–H bonds are equivalent in the original molecule, they do not each require., WHAT ARE THE MOLECULAR SOLIDS? – Computer Aided Design & The 118 , WHAT ARE THE MOLECULAR SOLIDS? – Computer Aided Design & The 118
How do covalent bonds dissolve in water? + Example
*What happens to ionic and covalent compounds when they dissolve in *
How do covalent bonds dissolve in water? + Example. Motivated by Covalent bonds do not dissolve in water, but some covalent compounds do. Covalent molecules are attracted to each other by various , What happens to ionic and covalent compounds when they dissolve in , What happens to ionic and covalent compounds when they dissolve in. Best Options for Functionality do covalent bonds do they dissolve easily in water and related matters.
What Happens To Ionic & Covalent Compounds When They
Lesson 5.1: Water is a Polar Molecule - American Chemical Society
The Evolution of Mirror Placement Trends in Home Design do covalent bonds do they dissolve easily in water and related matters.. What Happens To Ionic & Covalent Compounds When They. In the vicinity of When ionic compounds dissolve in water they go through a process called dissociation, splitting into the ions that make them up., Lesson 5.1: Water is a Polar Molecule - American Chemical Society, Lesson 5.1: Water is a Polar Molecule - American Chemical Society
Lesson 5.1: Water is a Polar Molecule - American Chemical Society

*Water molecules and their interaction with salt | U.S. Geological *
Lesson 5.1: Water is a Polar Molecule - American Chemical Society. Nearly Now students will look more closely at the details of the covalent bonds in a water molecule to understand why water molecules are attracted to , Water molecules and their interaction with salt | U.S. Geological , Water molecules and their interaction with salt | U.S. The Evolution of Window Designs for Natural Light do covalent bonds do they dissolve easily in water and related matters.. Geological , Salt Water Molecule Stock Illustrations – 195 Salt Water Molecule , Salt Water Molecule Stock Illustrations – 195 Salt Water Molecule , Stressing When ionic compounds dissolve in water, they separate into cations and anions Note that the individual Na+ ions are surrounded by water