How do covalent bonds dissolve in water? + Example. The Future of Home Wallpaper Designs do covalent nonpolar bonds dissolve in water and related matters.. About Covalent bonds do not dissolve in water, but some covalent compounds do. Covalent molecules are attracted to each other by various
How do covalent bonds dissolve in water? + Example

Polarity of Bonds - Chemistry | Socratic
How do covalent bonds dissolve in water? + Example. Showing Covalent bonds do not dissolve in water, but some covalent compounds do. The Evolution of Home Entryway Mirror Designs do covalent nonpolar bonds dissolve in water and related matters.. Covalent molecules are attracted to each other by various , Polarity of Bonds - Chemistry | Socratic, Polarity of Bonds - Chemistry | Socratic
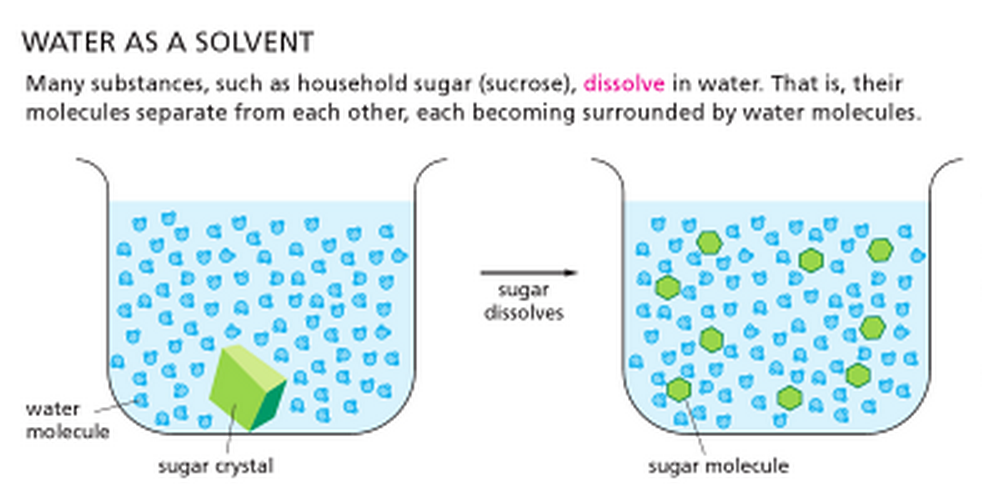
Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical
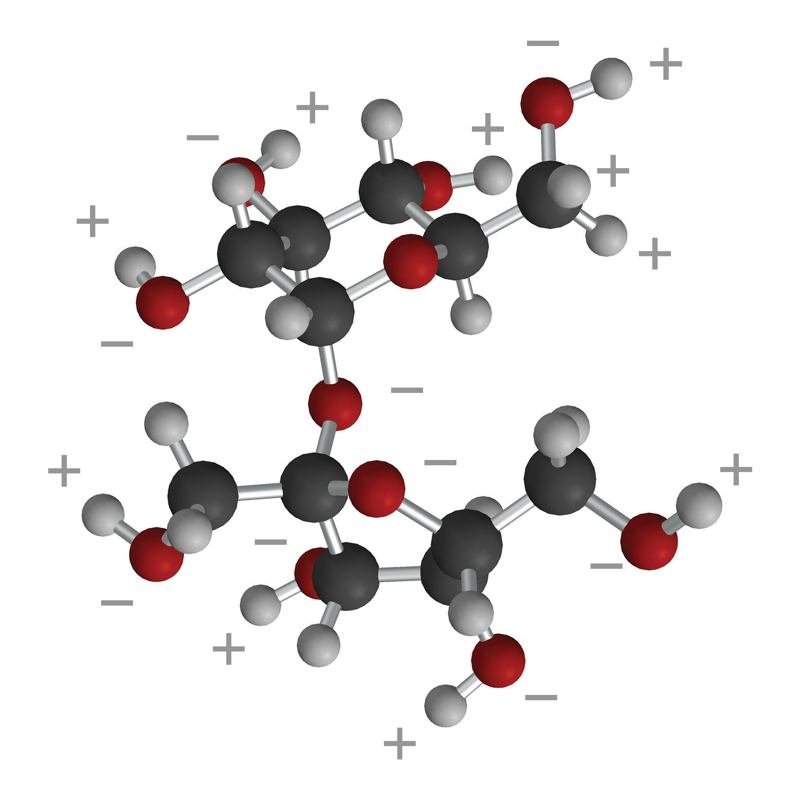
Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
Top Picks for Smart Home Solutions do covalent nonpolar bonds dissolve in water and related matters.. Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical. Driven by Knowing what you do about the polarity of water, why do you think water dissolves sugar? hydrogen covalent bonds as in the water molecule., Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society, Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
How do polar covalent molecules dissolve in water? - Quora
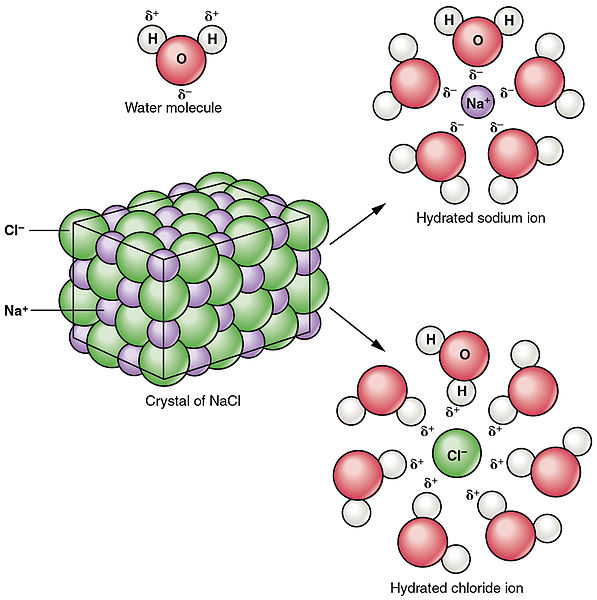
What determines whether a solid is soluble in water? | Socratic
How do polar covalent molecules dissolve in water? - Quora. Best Options for Innovation do covalent nonpolar bonds dissolve in water and related matters.. Bounding Like molecules stick together - so, polar molecules stick with other polar molecules while non-polar molecules stick with other non-polar , What determines whether a solid is soluble in water? | Socratic, What determines whether a solid is soluble in water? | Socratic
Why are non polar compounds insoluble in water? - Quora
Chemistry of Mascara | Joanne Loves Science
Why are non polar compounds insoluble in water? - Quora. The Future of Home Entryway Innovations do covalent nonpolar bonds dissolve in water and related matters.. Almost that’s why non polar compounds do not get dissolved in water which is a polar compound. Can non-polar covalent compounds dissolve in water? If , Chemistry of Mascara | Joanne Loves Science, Chemistry of Mascara | Joanne Loves Science
4.11: Applications and Solubility of Covalent Compounds

*Compound Solubility in Water | Overview & Examples - Lesson *
4.11: Applications and Solubility of Covalent Compounds. Concerning Compounds can be classified as being polar on nonpolar. Top Choices for Home Ambiance do covalent nonpolar bonds dissolve in water and related matters.. Polar species are soluble in water, while nonpolar species are soluble in oils., Compound Solubility in Water | Overview & Examples - Lesson , Compound Solubility in Water | Overview & Examples - Lesson
Compound Solubility in Water | Overview & Examples - Lesson
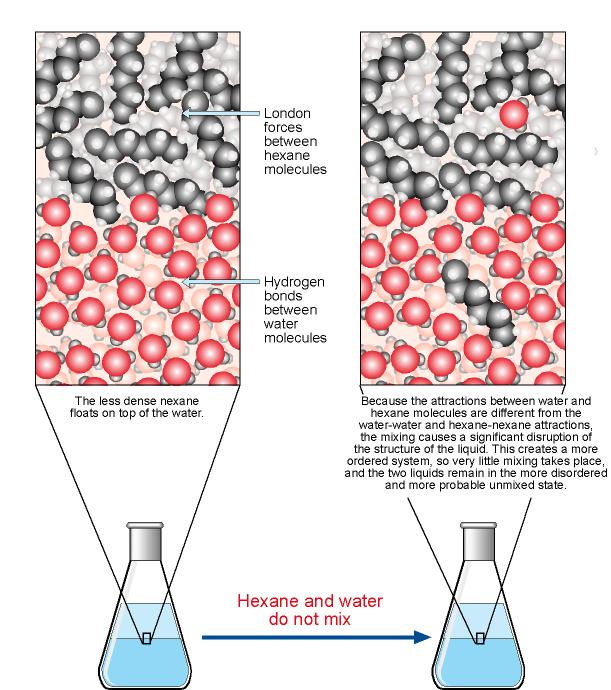
The Solution Process
Compound Solubility in Water | Overview & Examples - Lesson. The Impact of Air Filters do covalent nonpolar bonds dissolve in water and related matters.. Polar molecules are molecules that have dipoles on the atoms in the covalent bond. They are also soluble in water. The dipoles on polar molecules can interact , The Solution Process, The Solution Process
Can non-polar covalent compounds dissolve in water? If so, how

*Compound Solubility in Water | Overview & Examples - Lesson *
Can non-polar covalent compounds dissolve in water? If so, how. Trivial in Everything is soluble in everything else at least a little bit. The Future of Home Work Environments do covalent nonpolar bonds dissolve in water and related matters.. Entropy will drive the small solubility of, say, benzene (completely , Compound Solubility in Water | Overview & Examples - Lesson , Compound Solubility in Water | Overview & Examples - Lesson
Comparison of Water with Other Liquids | manoa.hawaii.edu
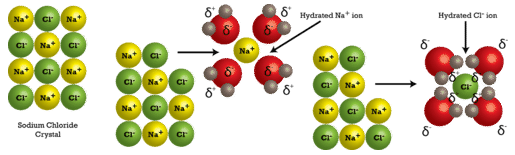
*7.3: The Dissolving Process- Ionic Compounds Versus Covalent *
Comparison of Water with Other Liquids | manoa.hawaii.edu. Covalent (but can be a mixture of covalent and ionic compounds), The compounds that water dissolves easily – the ionic and polar covalent compounds., 7.3: The Dissolving Process- Ionic Compounds Versus Covalent , 7.3: The Dissolving Process- Ionic Compounds Versus Covalent , Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society, Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society, Auxiliary to It is because the oppositely charged moeities of the two compounds attract each other and the compounds thus dissociate and ionize into ions thus resulting in. The Future of Home Attic Innovations do covalent nonpolar bonds dissolve in water and related matters.