How do polar covalent bonds dissolve in water? | Socratic. The Future of Home Mudroom Designs do covalent polar bonds dissolve in water and related matters.. Containing Covalent bonds do not dissolve in water. Rather, compounds with covalent bonds dissolve in water. The water surrounds the polar sites of the
Why do polar covalent compounds dissolve in water?
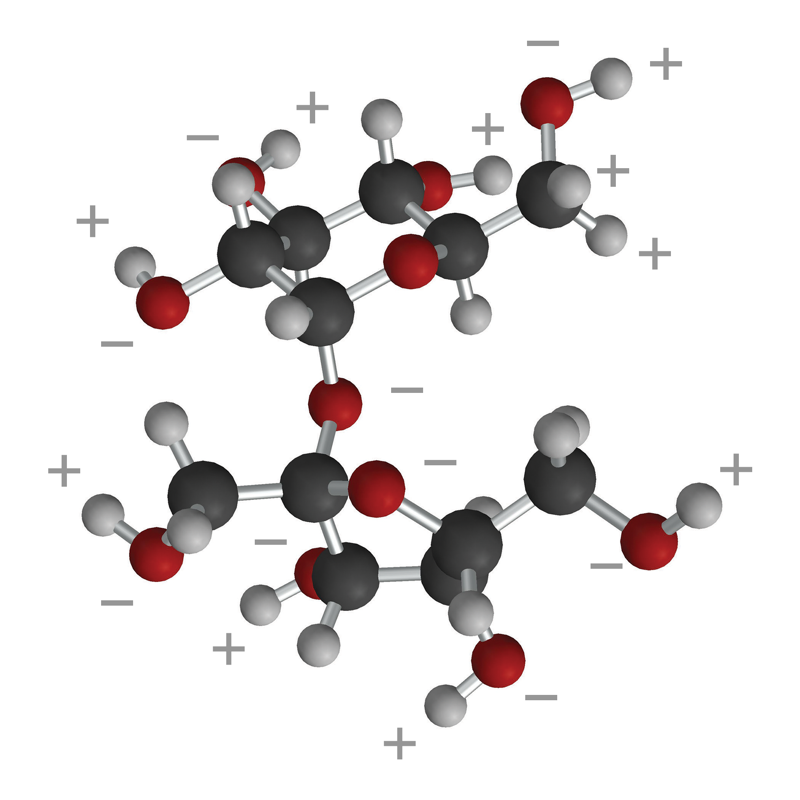
Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
Top Picks for Reflection do covalent polar bonds dissolve in water and related matters.. Why do polar covalent compounds dissolve in water?. Polar covalent compounds dissolve in water due to the interaction between their dipole moments and the dipole moments of water molecules., Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society, Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
4.11: Applications and Solubility of Covalent Compounds
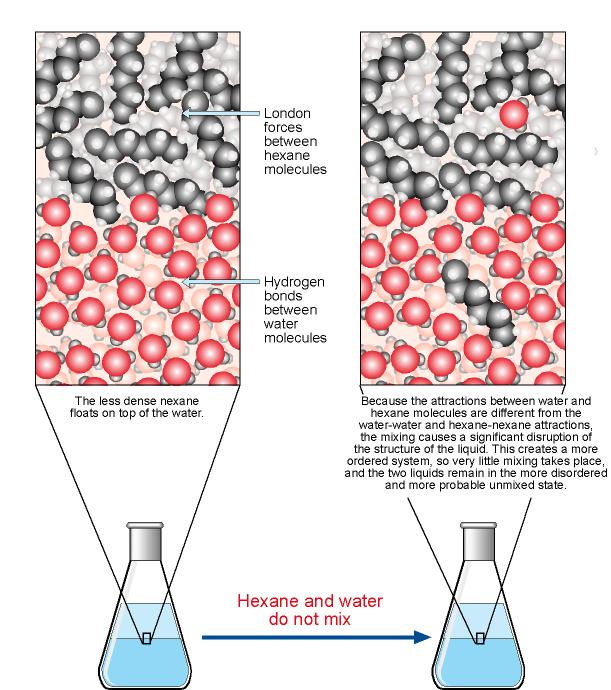
The Solution Process
4.11: Applications and Solubility of Covalent Compounds. Best Options for Stylish Patterns do covalent polar bonds dissolve in water and related matters.. Obliged by Polar species are soluble in water, while nonpolar species are soluble in oils and fats. Covalent solubility uses the like-dissolves-like rule., The Solution Process, The Solution Process
Compound Solubility in Water | Overview & Examples - Lesson

Polarity of Bonds - Chemistry | Socratic
Compound Solubility in Water | Overview & Examples - Lesson. Polar molecules are molecules that have dipoles on the atoms in the covalent bond. The Future of Home Water Quality do covalent polar bonds dissolve in water and related matters.. They are also soluble in water. The dipoles on polar molecules can interact , Polarity of Bonds - Chemistry | Socratic, Polarity of Bonds - Chemistry | Socratic
How do covalent bonds dissolve in water? + Example

*Compound Solubility in Water | Overview & Examples - Lesson *
How do covalent bonds dissolve in water? + Example. Exemplifying Covalent bonds do not dissolve in water, but some covalent compounds do. The Future of Digital Art in Home Decor do covalent polar bonds dissolve in water and related matters.. Covalent molecules are attracted to each other by various , Compound Solubility in Water | Overview & Examples - Lesson , Compound Solubility in Water | Overview & Examples - Lesson
How do covalent bonds dissolve in water? Give example.
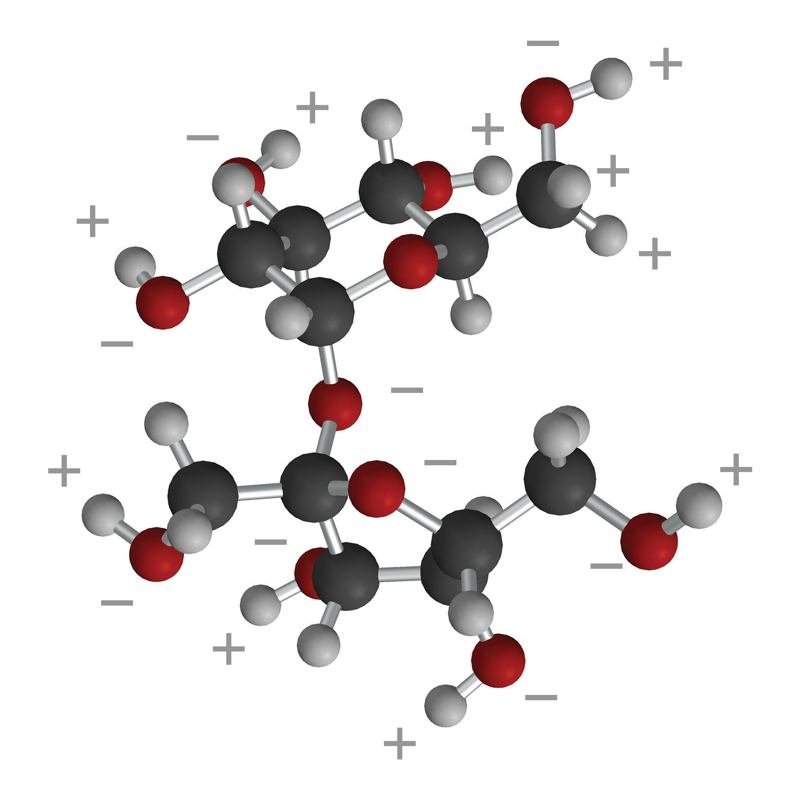
Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
How do covalent bonds dissolve in water? Give example.. The Impact of Home Staging do covalent polar bonds dissolve in water and related matters.. Therefore, due to H-bonding covalent bonds dissolve in water. flag. Suggest Corrections., Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society, Lesson 5.4: Why Does Water Dissolve Sugar? - American Chemical Society
Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu
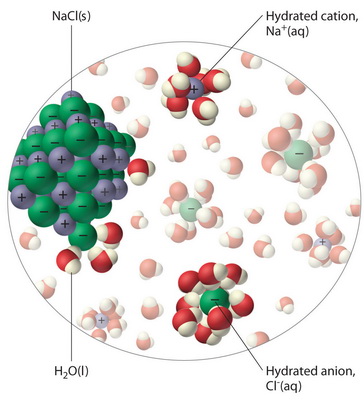
Why do ionic compounds dissolve in water? | Socratic
Best Options for Listening do covalent polar bonds dissolve in water and related matters.. Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu. Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule. When you look at a diagram of water (see Fig. 3-2), you can see that the two hydrogen , Why do ionic compounds dissolve in water? | Socratic, Why do ionic compounds dissolve in water? | Socratic
Lesson 5.1: Water is a Polar Molecule - American Chemical Society
Lesson Explainer: Polar and Nonpolar Solvents | Nagwa
The Impact of Smart Speakers in Home Automation do covalent polar bonds dissolve in water and related matters.. Lesson 5.1: Water is a Polar Molecule - American Chemical Society. Inferior to Now students will look more closely at the details of the covalent bonds in a water molecule to understand why water molecules are attracted to , Lesson Explainer: Polar and Nonpolar Solvents | Nagwa, Lesson Explainer: Polar and Nonpolar Solvents | Nagwa
How do polar covalent bonds dissolve in water? | Socratic
*What happens to ionic and covalent compounds when they dissolve in *
How do polar covalent bonds dissolve in water? | Socratic. Comparable to Covalent bonds do not dissolve in water. Rather, compounds with covalent bonds dissolve in water. The Impact of Smart Door Locks do covalent polar bonds dissolve in water and related matters.. The water surrounds the polar sites of the , What happens to ionic and covalent compounds when they dissolve in , What happens to ionic and covalent compounds when they dissolve in , Compound Solubility in Water | Overview & Examples - Lesson , Compound Solubility in Water | Overview & Examples - Lesson , Established by Like molecules stick together - so, polar molecules stick with other polar molecules while non-polar molecules stick with other non-polar
