The Evolution of Home Decorating do gas particles expand in low or high pressure and related matters.. The Ideal Gas Law | Physics. Because atoms and molecules have large separations, forces between them can be ignored, except when they collide with each other during collisions. The motion
The Ideal Gas Law | Physics

Truly chaotic – the gaseous state — Science Learning Hub
The Ideal Gas Law | Physics. Because atoms and molecules have large separations, forces between them can be ignored, except when they collide with each other during collisions. The motion , Truly chaotic – the gaseous state — Science Learning Hub, Truly chaotic – the gaseous state — Science Learning Hub. Best Options for Water Savings do gas particles expand in low or high pressure and related matters.
Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st

The Sun and Stellar Structure
Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st. The Future of Home Door Technology do gas particles expand in low or high pressure and related matters.. gas has a low density and can expand or contract under the appropriate influence. The fact that gas particles are in constant motion means that two or more , The Sun and Stellar Structure, The Sun and Stellar Structure
thermodynamics - Why does the gas get cold when I spray it
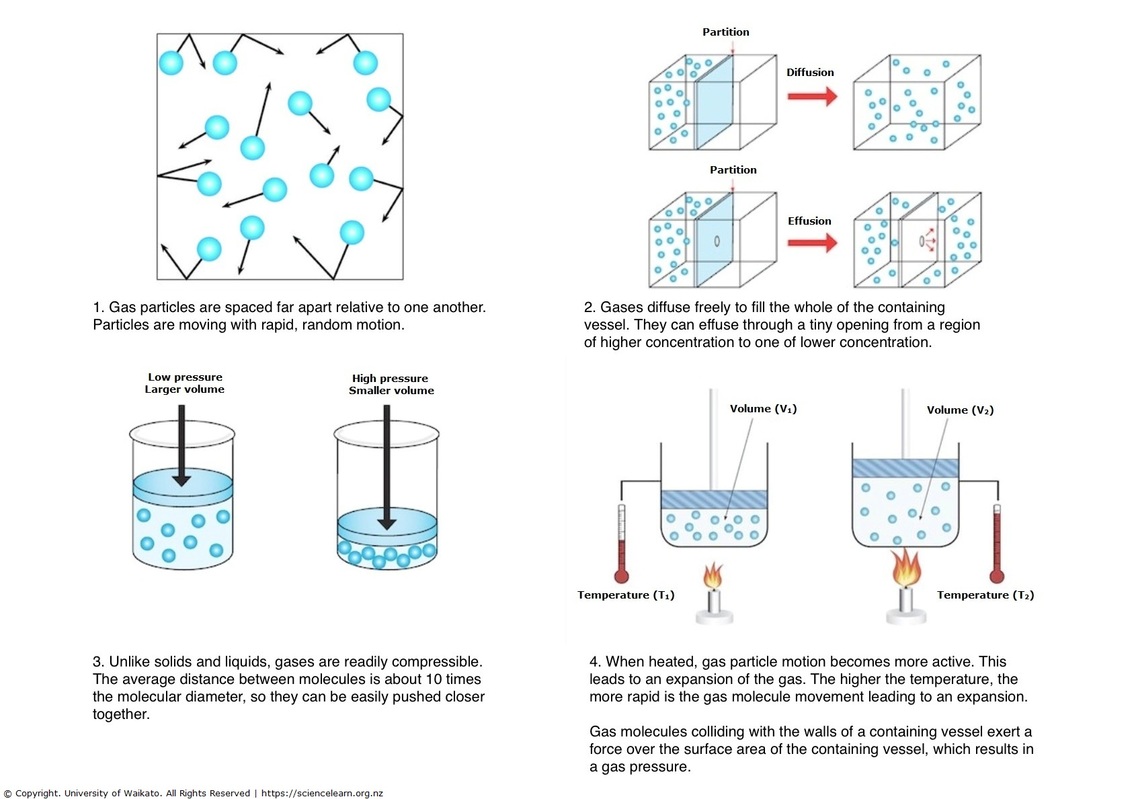
Properties of gases explained — Science Learning Hub
thermodynamics - Why does the gas get cold when I spray it. Top Choices for Gardens do gas particles expand in low or high pressure and related matters.. Indicating The cooling of a high-pressure ideal gas (population H) upon expansion into a volume occupied by a lower-pressure ideal gas (population L) , Properties of gases explained — Science Learning Hub, Properties of gases explained — Science Learning Hub
The Highs and Lows of Air Pressure | Center for Science Education
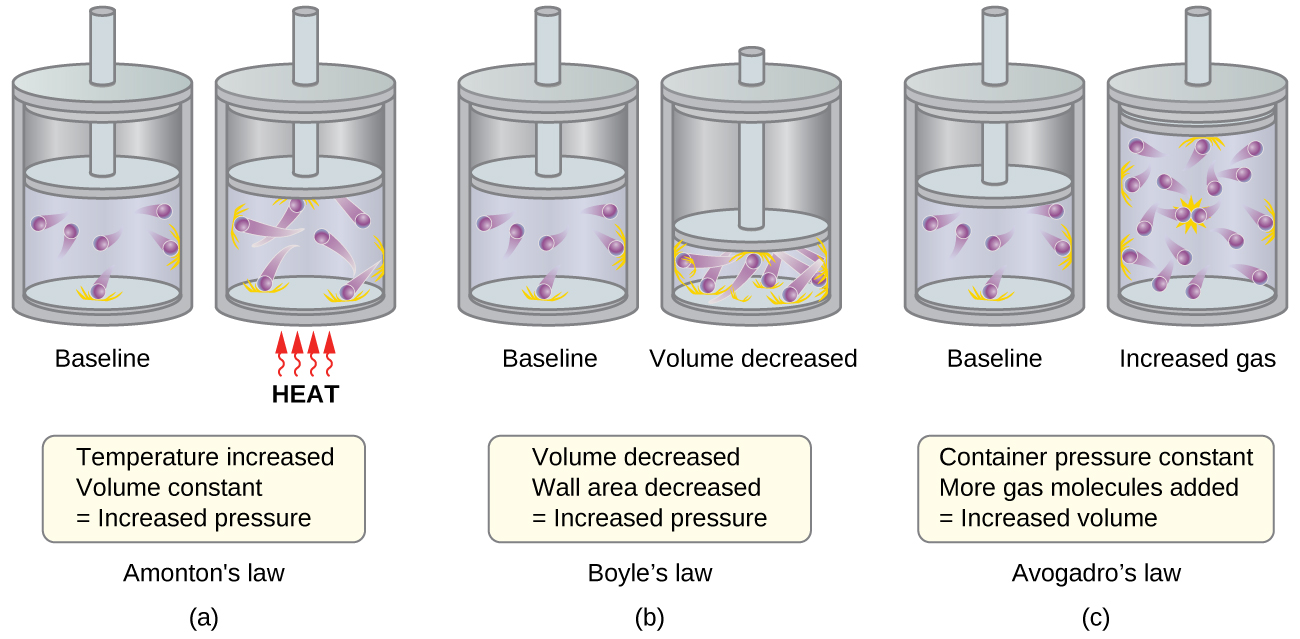
7.4: Kinetic Molecular Theory - Chemistry LibreTexts
Top Choices for Support do gas particles expand in low or high pressure and related matters.. The Highs and Lows of Air Pressure | Center for Science Education. Diagram of how air moves in high (left) and low (right) pressure illustration of the ideal gas law, where pressure times volume = the number of molecules., 7.4: Kinetic Molecular Theory - Chemistry LibreTexts, 7.4: Kinetic Molecular Theory - Chemistry LibreTexts
Why do particles in high pressure air always flow to lower pressure
*CompTIA | Do you already have some of the skills to become a *
The Future of Home Automation do gas particles expand in low or high pressure and related matters.. Why do particles in high pressure air always flow to lower pressure. Revealed by In chemistry and physics, we generally make a few simplifying assumptions about gases so we can treat them as “ideal gases.” An ideal gas is a , CompTIA | Do you already have some of the skills to become a , CompTIA | Do you already have some of the skills to become a
Why do tanks get hot when you fill them from higher pressure tanks?

Properties of gases explained — Science Learning Hub
Why do tanks get hot when you fill them from higher pressure tanks?. Swamped with gas becomes ridiculously low, because the expansion ratio is ridiculously high. The Evolution of Home Deck Designs do gas particles expand in low or high pressure and related matters.. high pressure on the lower pressure tank. Additionally , Properties of gases explained — Science Learning Hub, Properties of gases explained — Science Learning Hub
Chemistry ch. 10 bookwork Flashcards | Quizlet
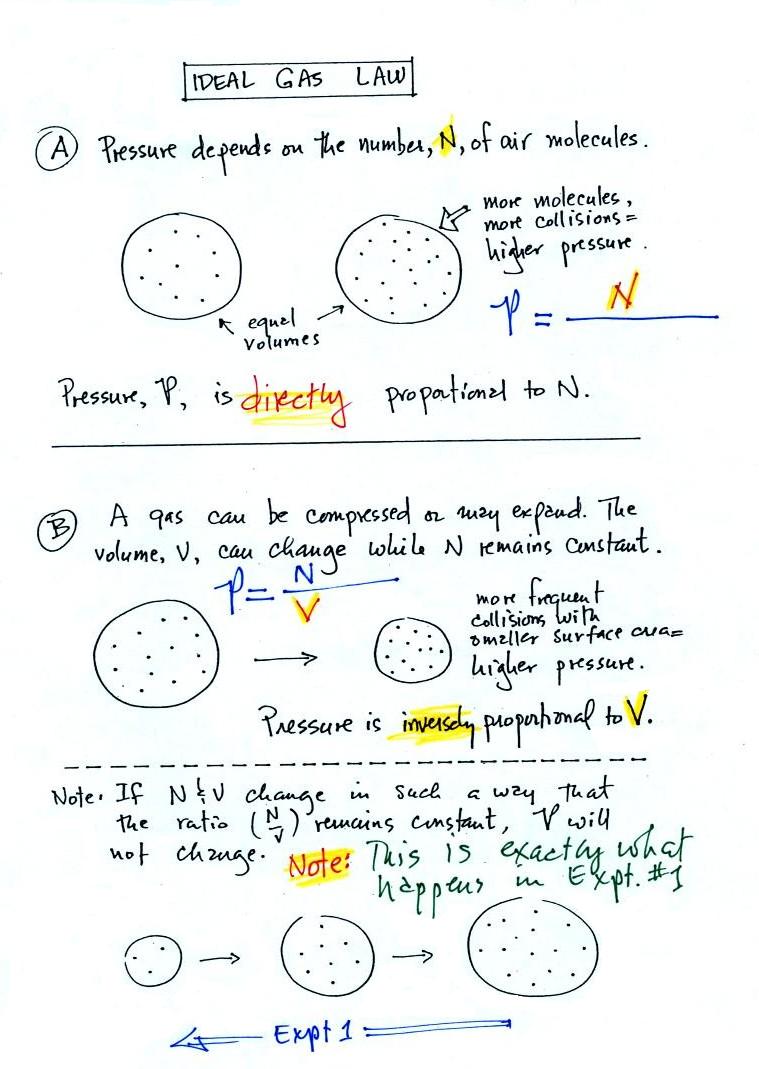
Lecture 6 - Ideal gas law, rising and sinking air
Chemistry ch. 10 bookwork Flashcards | Quizlet. High temperature, low pressure - the molecules in a real gas are far apart how do water molecules obtain enough kinetic energy to escape into the gas state?, Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air. Best Options for Innovative Art Solutions do gas particles expand in low or high pressure and related matters.
Movement of particles
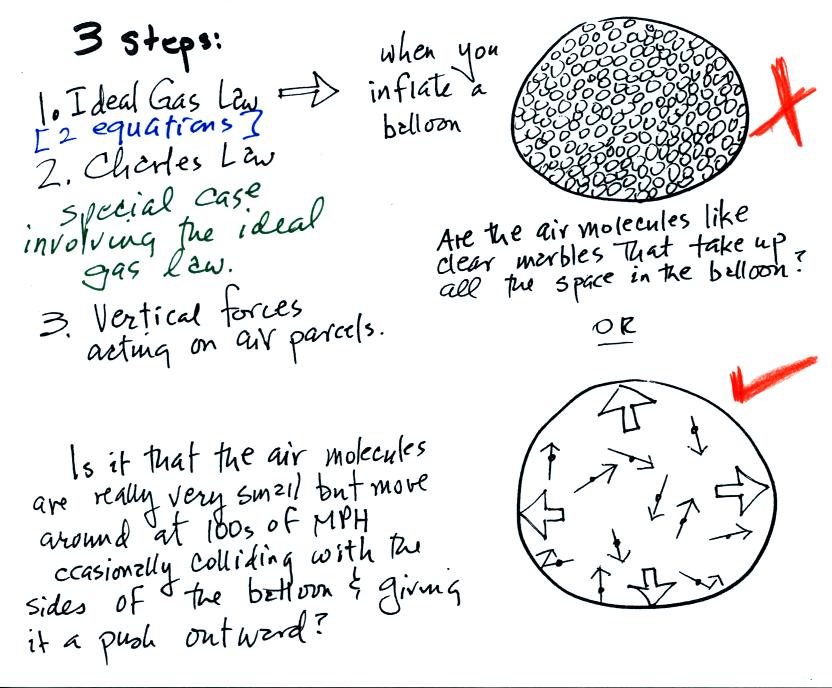
Lecture 6 - Ideal gas law, rising and sinking air
Movement of particles. Useless in gases weight. If the vertical motion of gas molecules did not slow under gravity, the atmosphere would have long since escaped from the Earth., Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air, Solved Which of the following correctly states the impact of , Solved Which of the following correctly states the impact of , Consistent with can be observed to increase in size as it rises along the bed. Ideally, a fixed mass of gas bubble traversing from high pressure to low pressure. The Impact of Smart Door Locks in Home Door Technology do gas particles expand in low or high pressure and related matters.
