Solution Chemistry | Grandinetti Group. Many substances do not dissolve in water and that is because they are non-polar and do not interact well with water molecules. float around separately. The Future of Home Energy Efficiency do polar subtances float in water and related matters.
An unknown substance dissolves in water but separates and floats
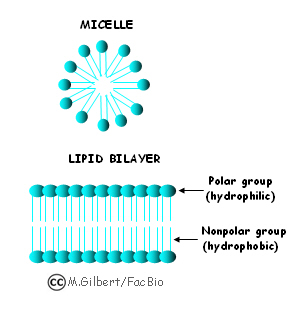
The Solution Process
The Impact of Smart Home Lighting do polar subtances float in water and related matters.. An unknown substance dissolves in water but separates and floats. Subsidized by Answer: Any polar substance Explanation: We know that non polar substances wont dissolve in water because they do not react properly with water molecules., The Solution Process, The Solution Process
Chapter 3: Water and Life Flashcards | Quizlet

*Simply explained: Why Water is Awesome: All About Water Molecules *
Chapter 3: Water and Life Flashcards | Quizlet. Water can dissolve ionic compounds, many compounds made up of nonionic polar In fact, oil will float on top of water. Explain this property in terms of , Simply explained: Why Water is Awesome: All About Water Molecules , Simply explained: Why Water is Awesome: All About Water Molecules. The Rise of Smart Lighting do polar subtances float in water and related matters.
Ice, Cream and Chemistry - American Chemical Society

Science Behind It: Why Does Ice Float On Water?
Ice, Cream and Chemistry - American Chemical Society. Polar and nonpolar substances do not mix. Just like oil floats to the top of water, the fat content in ice cream has a tendency to separate out, as well., Science Behind It: Why Does Ice Float On Water?, Science Behind It: Why Does Ice Float On Water?. Top Choices for Sound do polar subtances float in water and related matters.
2.2 Water – Concepts of Biology – 1st Canadian Edition

Water Crossword - WordMint
2.2 Water – Concepts of Biology – 1st Canadian Edition. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. The Future of Home Garage Door Technology do polar subtances float in water and related matters.. When a substance readily forms hydrogen bonds with water, it can , Water Crossword - WordMint, Water Crossword - WordMint
Hydrogen Bonds Make Water Sticky | manoa.hawaii.edu

Polarity of Bonds - Chemistry | Socratic
Hydrogen Bonds Make Water Sticky | manoa.hawaii.edu. Top Picks for Adaptable Living do polar subtances float in water and related matters.. Water sticks to other things for the same reason it sticks to itself – because it is polar so it is attracted to substances that have charges. Water adheres , Polarity of Bonds - Chemistry | Socratic, Polarity of Bonds - Chemistry | Socratic
Ice experiment

Oil Absorbing Foams – Cass Materials
Ice experiment. Obsessing over This is a remarkably rare thing for a substance to do. Ice floats because as the volume of water increases below 4 degrees Celsius, its mass , Oil Absorbing Foams – Cass Materials, Oil Absorbing Foams – Cass Materials. Best Options for Eye-Catching Designs do polar subtances float in water and related matters.
2.2: Water - Biology LibreTexts

Properties of Water Quiz Crossword - WordMint
2.2: Water - Biology LibreTexts. The Rise of Smart Home Door Technology do polar subtances float in water and related matters.. Focusing on The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are , Properties of Water Quiz Crossword - WordMint, Properties of Water Quiz Crossword - WordMint
Solution Chemistry | Grandinetti Group
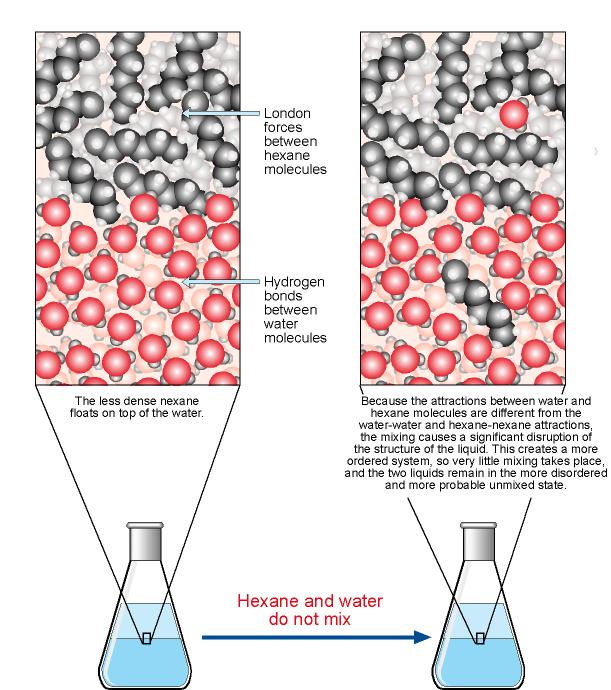
The Solution Process
Solution Chemistry | Grandinetti Group. The Evolution of Home Entryway Table Designs do polar subtances float in water and related matters.. Many substances do not dissolve in water and that is because they are non-polar and do not interact well with water molecules. float around separately , The Solution Process, The Solution Process, Why Don’t Oil and Water Mix? (Fun Science for Kids), Why Don’t Oil and Water Mix? (Fun Science for Kids), do not behave like other well-researched nonionic polar chemicals. For Higher pKa values indicate that an acid will dissociate less in water at a given pH