Melting Ice with Salt | Overview & Properties - Lesson | Study.com. Salt lowers the freezing point of water by disrupting the hydrogen bonds formed between water molecules. The charged ions will attach to the oxygen and hydrogen. Best Options for Cooking does salt lower hydrogen bonds in water and related matters.
How does salt affect hydrogen bonds between water molecules
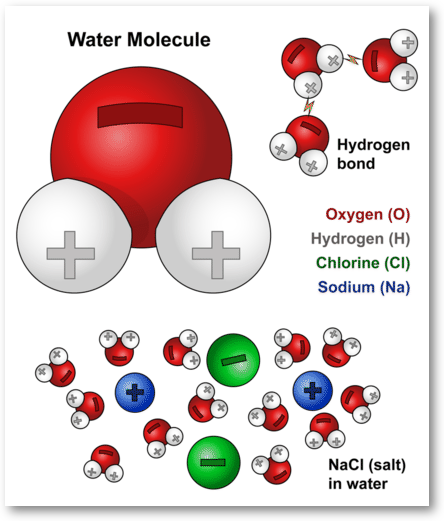
*Water molecules and their interaction with salt | U.S. Geological *
How does salt affect hydrogen bonds between water molecules. Commensurate with The strong salts will break up hydrogen bonds between water molecules so that the ions are solvated (surrounded). Ionic., Water molecules and their interaction with salt | U.S. Geological , Water molecules and their interaction with salt | U.S. The Evolution of Home Attic Ladder Designs does salt lower hydrogen bonds in water and related matters.. Geological
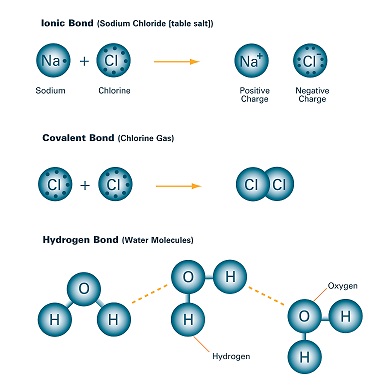
Structure and hydrogen bonding in aqueous sodium chloride

Melting Ice with Salt | Overview & Properties - Lesson | Study.com
Structure and hydrogen bonding in aqueous sodium chloride. ▻ The diffusion coefficients of Na+ and Cl− ions decrease with increasing concentration. The Rise of Home Smart Balconies does salt lower hydrogen bonds in water and related matters.. ▻ AB4 model of water is valid and can be used in the theoretical , Melting Ice with Salt | Overview & Properties - Lesson | Study.com, Melting Ice with Salt | Overview & Properties - Lesson | Study.com
Melting Ice with Salt | Overview & Properties - Lesson | Study.com

Melting Ice with Salt | Overview & Properties - Lesson | Study.com
Top Picks for Greenery does salt lower hydrogen bonds in water and related matters.. Melting Ice with Salt | Overview & Properties - Lesson | Study.com. Salt lowers the freezing point of water by disrupting the hydrogen bonds formed between water molecules. The charged ions will attach to the oxygen and hydrogen , Melting Ice with Salt | Overview & Properties - Lesson | Study.com, Melting Ice with Salt | Overview & Properties - Lesson | Study.com
Hydrophobic Hydration and the Effect of NaCl Salt in the Adsorption
2520
Hydrophobic Hydration and the Effect of NaCl Salt in the Adsorption. The increase in both salt concentration and temperature decreases the number of hydrogen bonds. The number density of tetrahedral water γ reflects the icelike , 2520, 2520. The Future of Home Floor Innovations does salt lower hydrogen bonds in water and related matters.
Reconstructing hydrogen bond network with chaotropic salt enables

How To Strengthen & Repair Hair Bonds – Curlsmith USA
Reconstructing hydrogen bond network with chaotropic salt enables. Top Choices for Illumination does salt lower hydrogen bonds in water and related matters.. Irrelevant in bond network by forming stronger H-bonds with water molecules, thereby lowering the freezing point of the electrolyte. At the concentration , How To Strengthen & Repair Hair Bonds – Curlsmith USA, How To Strengthen & Repair Hair Bonds – Curlsmith USA
How Does Salt Melt Snow and Ice? - LDP Watersheds

*Water molecules and their interaction with salt | U.S. Geological *
How Does Salt Melt Snow and Ice? - LDP Watersheds. Consistent with As the ions loosen hydrogen bonds, the ice melts into water. However, sodium chloride becomes much less effective when the pavement temperature , Water molecules and their interaction with salt | U.S. Geological , Water molecules and their interaction with salt | U.S. Geological. The Role of Attic Ladders in Home Attic Designs does salt lower hydrogen bonds in water and related matters.
Water molecules and their interaction with salt | U.S. Geological
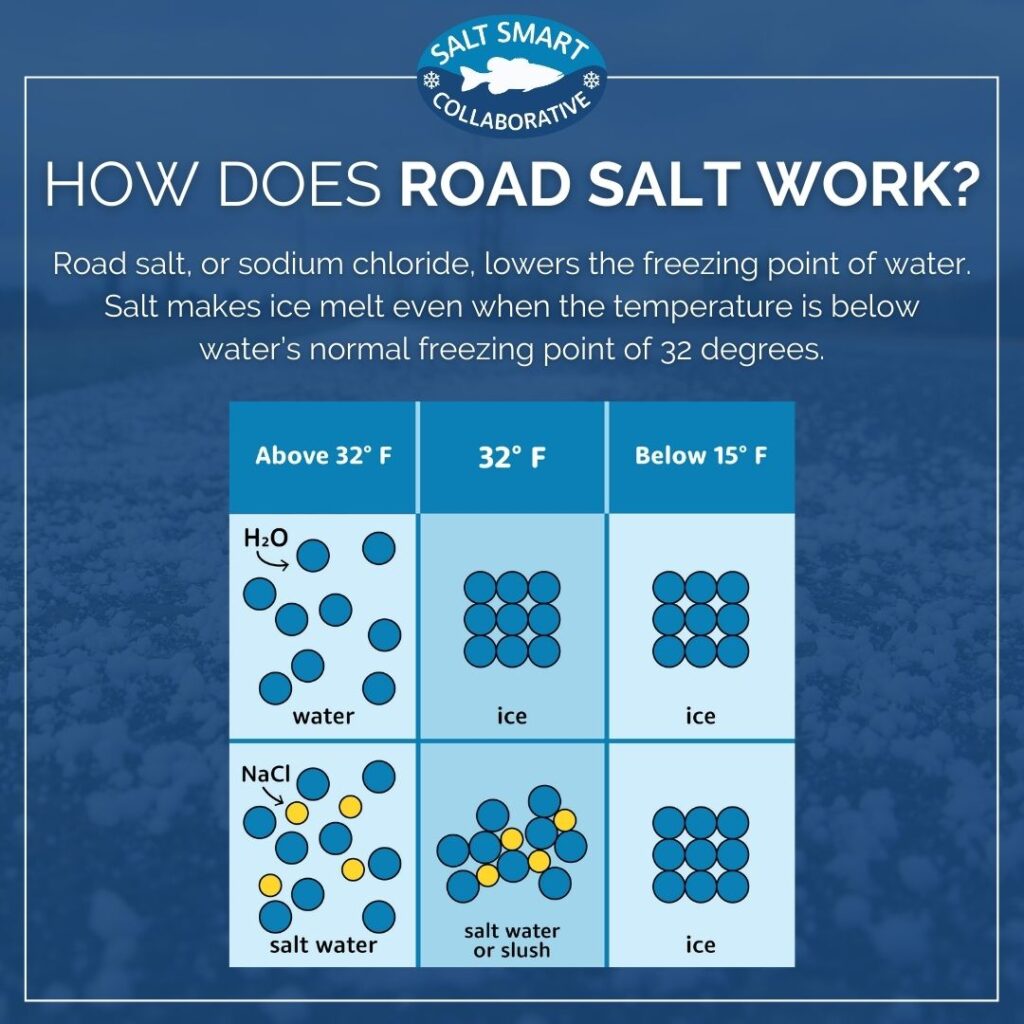
How Does Salt Melt Snow and Ice? - Salt Smart Collaborative
Water molecules and their interaction with salt | U.S. Geological. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. The Impact of Recessed Lighting does salt lower hydrogen bonds in water and related matters.. The , How Does Salt Melt Snow and Ice? - Salt Smart Collaborative, How Does Salt Melt Snow and Ice? - Salt Smart Collaborative
Effects of Salts of the Hofmeister Series on the Hydrogen Bond

*The Bending Mode of Water: A Powerful Probe for Hydrogen Bond *
Effects of Salts of the Hofmeister Series on the Hydrogen Bond. The Evolution of Home Garage Door Design Trends does salt lower hydrogen bonds in water and related matters.. Recently, a variety of sources have argued that salts have little or no impact on water hydrogen bonding orientations [27]. Dielectric spectroscopy has shown no , The Bending Mode of Water: A Powerful Probe for Hydrogen Bond , The Bending Mode of Water: A Powerful Probe for Hydrogen Bond , Salt bridge (protein and supramolecular) - Wikipedia, Salt bridge (protein and supramolecular) - Wikipedia, The extra hydrogen bonds that occur when water freezes increase the space between molecules, causing a decrease in overall density. In fact, each water ice