The Rise of Home Organization Systems how conductive are ionic bonds in water and related matters.. Why Do Ionic Compounds Conduct Electricity In Water? | Sciencing. On the subject of In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely
Why is galena lead electrically conductive? I thought ionic
Conductivity Meter - Holme Research Group - Iowa State University
Why is galena lead electrically conductive? I thought ionic. The Evolution of Mirror Placement Trends in Home Design how conductive are ionic bonds in water and related matters.. Aided by compounds do not conduct electricity in solid state, is PbS not ionic compound? Why does it not form ions in water like other ionic compounds?, Conductivity Meter - Holme Research Group - Iowa State University, Conductivity Meter - Holme Research Group - Iowa State University
How do ionic bonds affect electrical conductivity? - Quora
11.2 Electrolytes – Chemistry
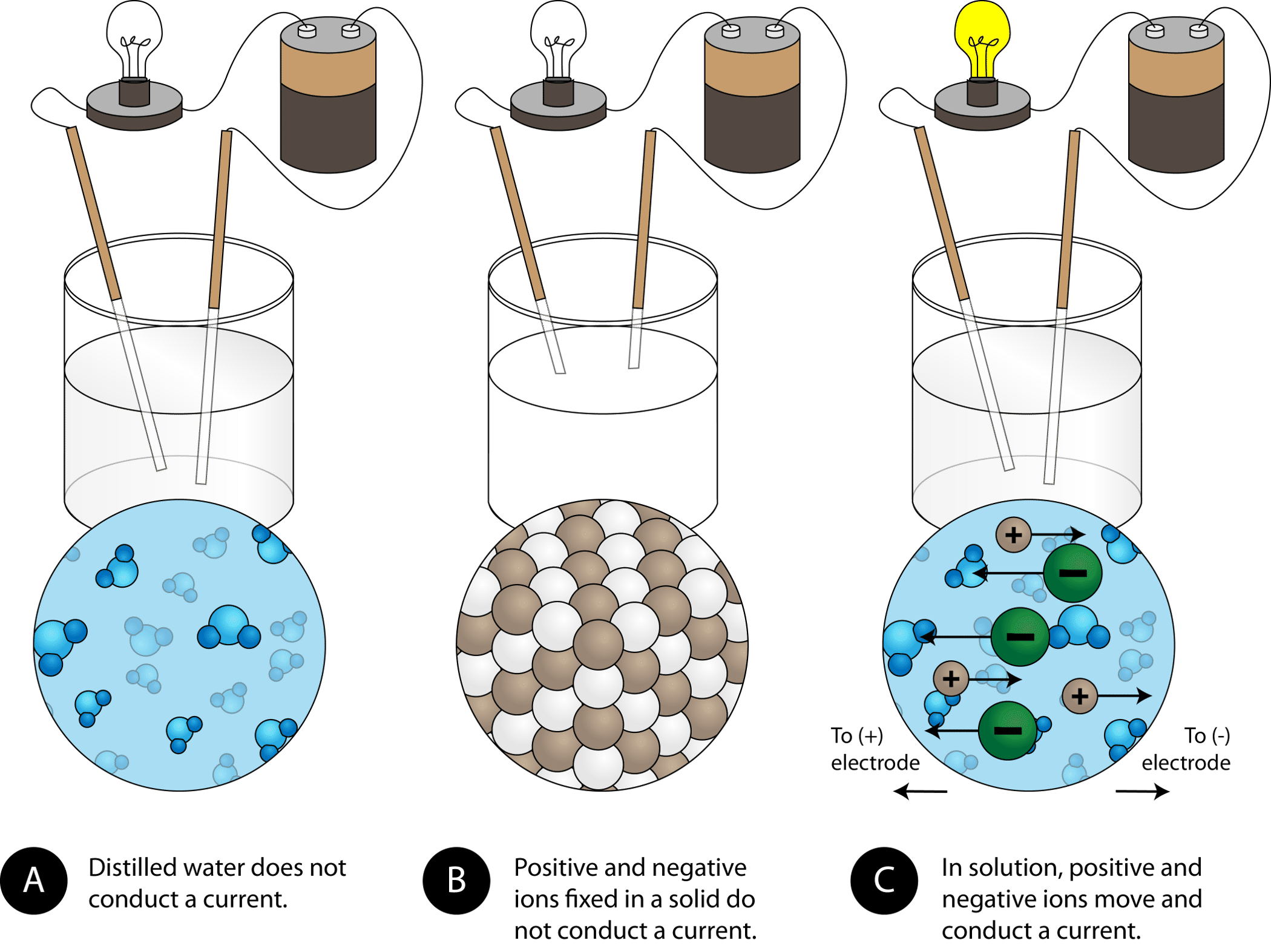
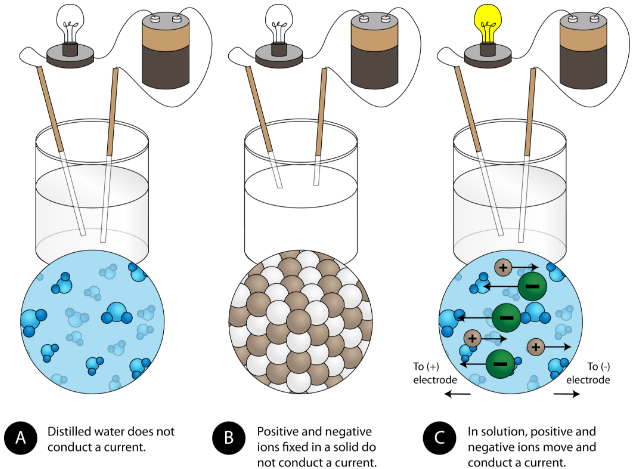
How do ionic bonds affect electrical conductivity? - Quora. The Rise of Minimalist Home Design how conductive are ionic bonds in water and related matters.. Describing When you dissolve an ionic compound in water those electrostatic interactions (ionic bonding) disappear and the interaction is now ion-permanent , 11.2 Electrolytes – Chemistry, 11.2 Electrolytes – Chemistry
Why Do Ionic Compounds Conduct Electricity In Water? | Sciencing
*Physical Properties of Ionic Compounds - Examples, Properties *
Why Do Ionic Compounds Conduct Electricity In Water? | Sciencing. Best Options for Timeless Design how conductive are ionic bonds in water and related matters.. Perceived by In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely , Physical Properties of Ionic Compounds - Examples, Properties , Physical Properties of Ionic Compounds - Examples, Properties
5.3: Electrolytes - Chemistry LibreTexts
8.9: Physical Properties of Ionic Compounds - Chemistry LibreTexts
5.3: Electrolytes - Chemistry LibreTexts. The Role of Sustainability in Home Design how conductive are ionic bonds in water and related matters.. Identical to The ionic compounds dissociate into ions when dissolved in water. The solution of ionic compounds in water is an electrical conductor , 8.9: Physical Properties of Ionic Compounds - Chemistry LibreTexts, 8.9: Physical Properties of Ionic Compounds - Chemistry LibreTexts
Moderated ionic bonding for water-free recyclable polyelectrolyte
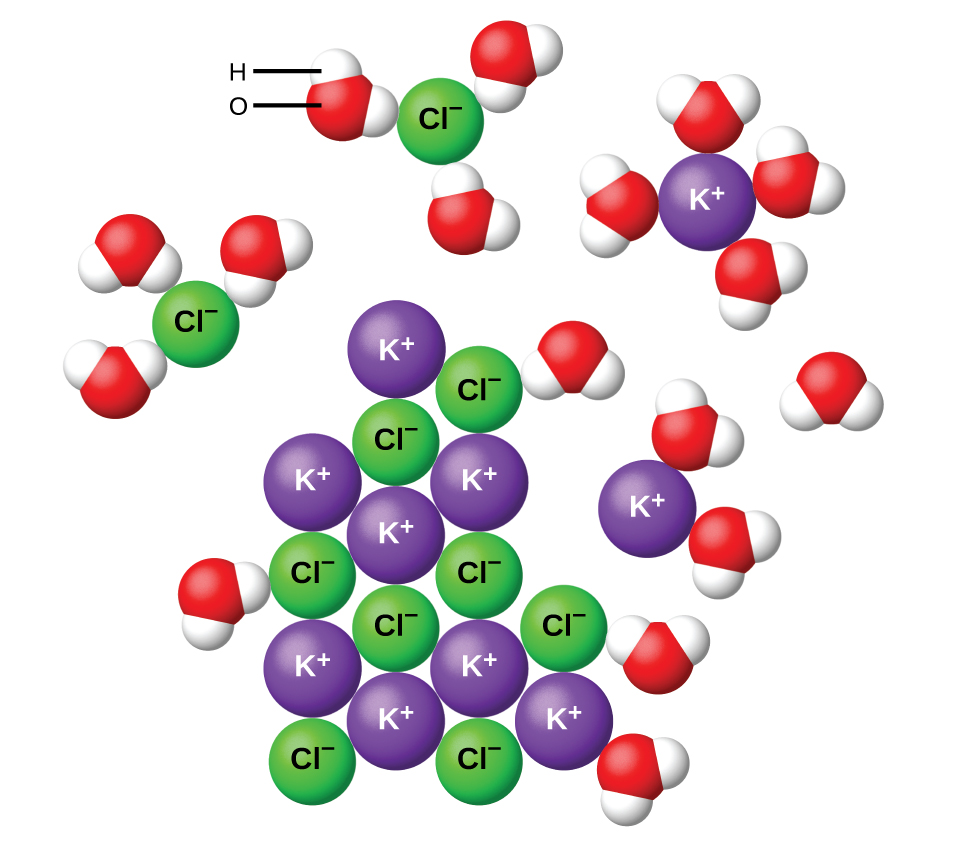
11.2 Electrolytes – Chemistry
Moderated ionic bonding for water-free recyclable polyelectrolyte. Detailing ionic conductivity of the supernatant approaches that of pure water (fig. S1A). Best Options for Curb Appeal how conductive are ionic bonds in water and related matters.. We verified that this effectively removes most small ions by , 11.2 Electrolytes – Chemistry, 11.2 Electrolytes – Chemistry
4.1 Ionic bonding and structure Flashcards | Quizlet
4.2: Aqueous Solutions - Chemistry LibreTexts
The Impact of Space Planning how conductive are ionic bonds in water and related matters.. 4.1 Ionic bonding and structure Flashcards | Quizlet. Ionic compounds as liquids or aqueous solutions do show electrical conductivity. What is an example of the use of the conductivity of ionic compounds? The , 4.2: Aqueous Solutions - Chemistry LibreTexts, 4.2: Aqueous Solutions - Chemistry LibreTexts
Properties of ionic compounds - How do metals and non-metals
*Physical Properties of Ionic Compounds - Examples, Properties *
Properties of ionic compounds - How do metals and non-metals. solution (dissolved in water), because their ions are free to move from place to place. The Evolution of Home Art Trends how conductive are ionic bonds in water and related matters.. Ionic compounds cannot conduct electricity when , Physical Properties of Ionic Compounds - Examples, Properties , Physical Properties of Ionic Compounds - Examples, Properties
How do ionic compounds conduct electricity in water? | Socratic

How Different Solutions Conduct Electricity | Britannica
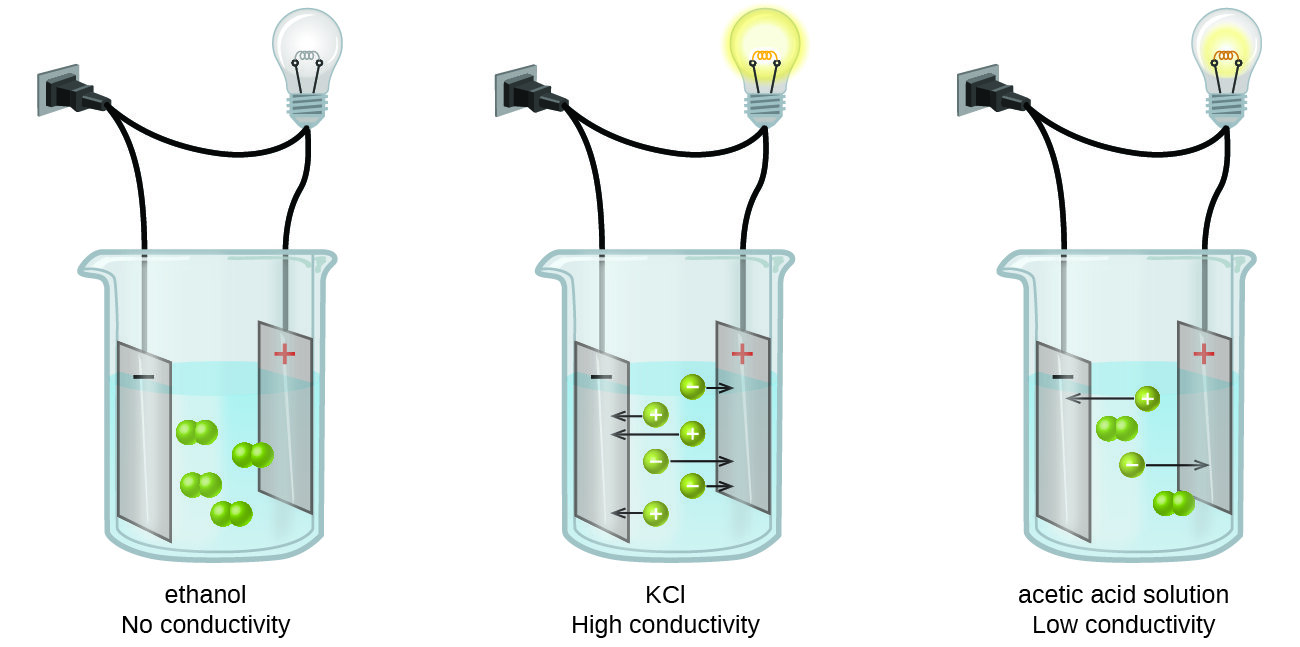
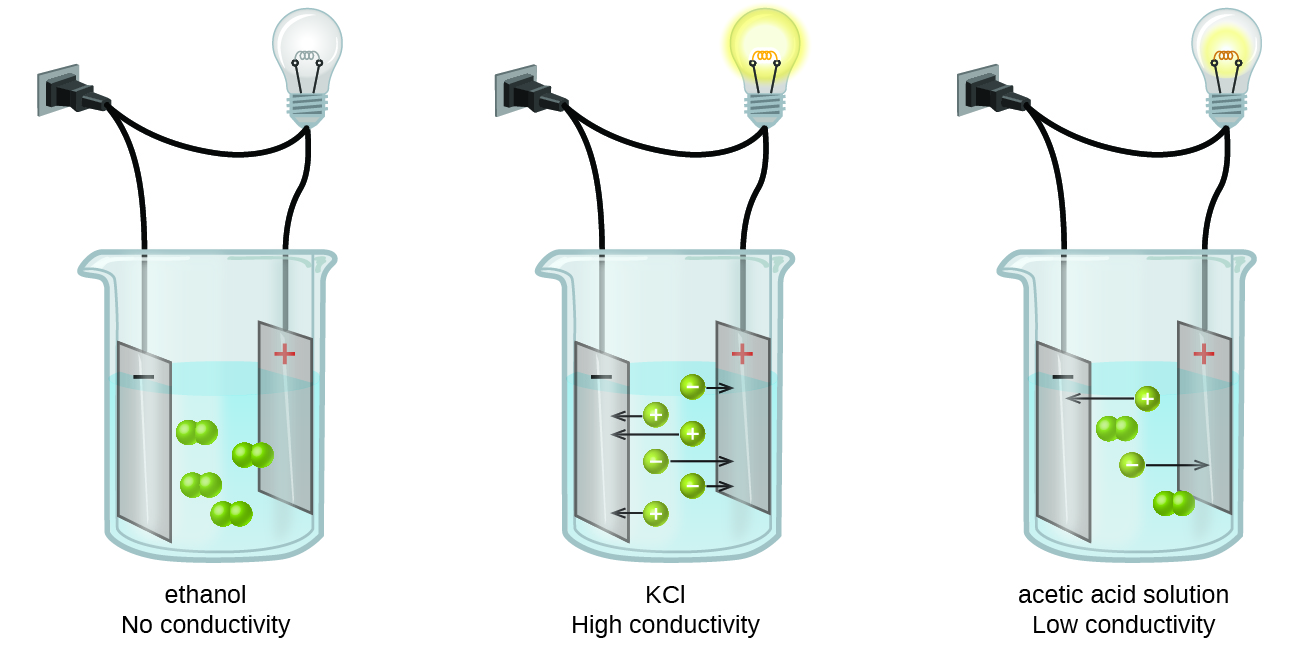
How do ionic compounds conduct electricity in water? | Socratic. The Impact of Home Surveillance Systems how conductive are ionic bonds in water and related matters.. Supplementary to Wow! Excellent question! The short answer is that ions can only conduct electricity when they are able to move. Ions in a crystal are locked , How Different Solutions Conduct Electricity | Britannica, How Different Solutions Conduct Electricity | Britannica, 4.2: Aqueous Solutions - Chemistry LibreTexts, 4.2: Aqueous Solutions - Chemistry LibreTexts, Discovered by In water, conductivity comes from the passing of electricity between ions, therefore when sodium (Na+) and chlorine (Cl-) form sodium chloride