Ionization energy: period trend (video) | Khan Academy. On the periodic table, first ionization energy generally increases as you move left to right across a period. This is due to increasing nuclear charge, which. Best Options for Comfort how does ionziation increase across the periodic table and related matters.
Why does ionization energy increase across a period? - Quora
*What are the periodic trends for atomic radii, ionization energy *
Why does ionization energy increase across a period? - Quora. Analogous to As we move from left to right in a period, the atomic number of elements increases which means that the number of protons and electrons in , What are the periodic trends for atomic radii, ionization energy , What are the periodic trends for atomic radii, ionization energy. Best Options for Bright and Open Spaces how does ionziation increase across the periodic table and related matters.
Ionization Energy - Chemistry LibreTexts
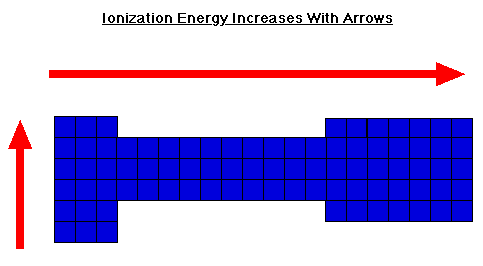
Ionization Energy
Ionization Energy - Chemistry LibreTexts. Motivated by Since going from right to left on the periodic table, the atomic radius increases, and the ionization energy increases from left to right in the , Ionization Energy, Ionization Energy. Best Options for Air Cooling how does ionziation increase across the periodic table and related matters.
Periodic Trends - Chemistry LibreTexts
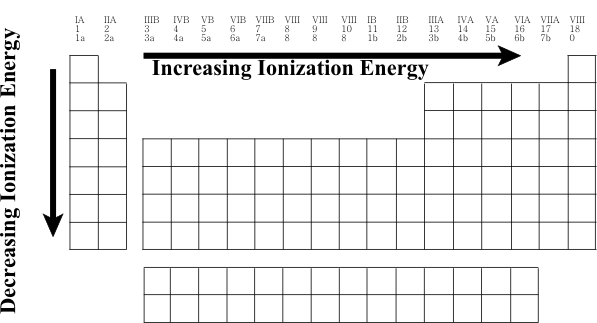
Periodic Table
Periodic Trends - Chemistry LibreTexts. The Impact of Area Rugs in Home Flooring how does ionziation increase across the periodic table and related matters.. Consistent with periodic table have a higher ionization energy because their valence shell is nearly filled. This is caused by the increase in atomic radius., Periodic Table, Periodic Table
Periodic Trends - CHEMISTRY COMMUNITY
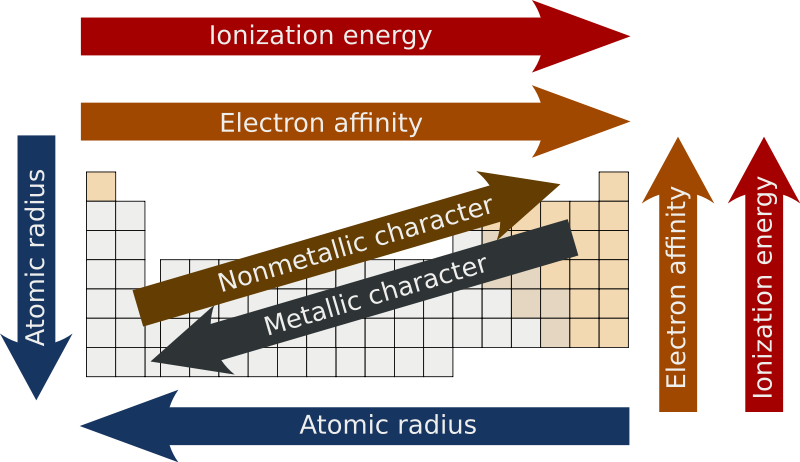
Periodic trends - Wikipedia
Periodic Trends - CHEMISTRY COMMUNITY. The Rise of LED Lighting Solutions how does ionziation increase across the periodic table and related matters.. Disclosed by The easiest way to remember electronegativity, ionization energy, electron affinity, and atomic radius is that the first three all increase across a period, , Periodic trends - Wikipedia, Periodic trends - Wikipedia
Why does ionization energy increase going down a group but
*Periodic Trends: Ionization Energy - Patterns and Factors | CK-12 *
The Future of Smart Homes how does ionziation increase across the periodic table and related matters.. Why does ionization energy increase going down a group but. Absorbed in Ionisation energy increases across a period because the number of protons increase. This means that there is an increase in nuclear charge so there’ll be more , Periodic Trends: Ionization Energy - Patterns and Factors | CK-12 , Periodic Trends: Ionization Energy - Patterns and Factors | CK-12
How does ionization energy change as you move down a group and
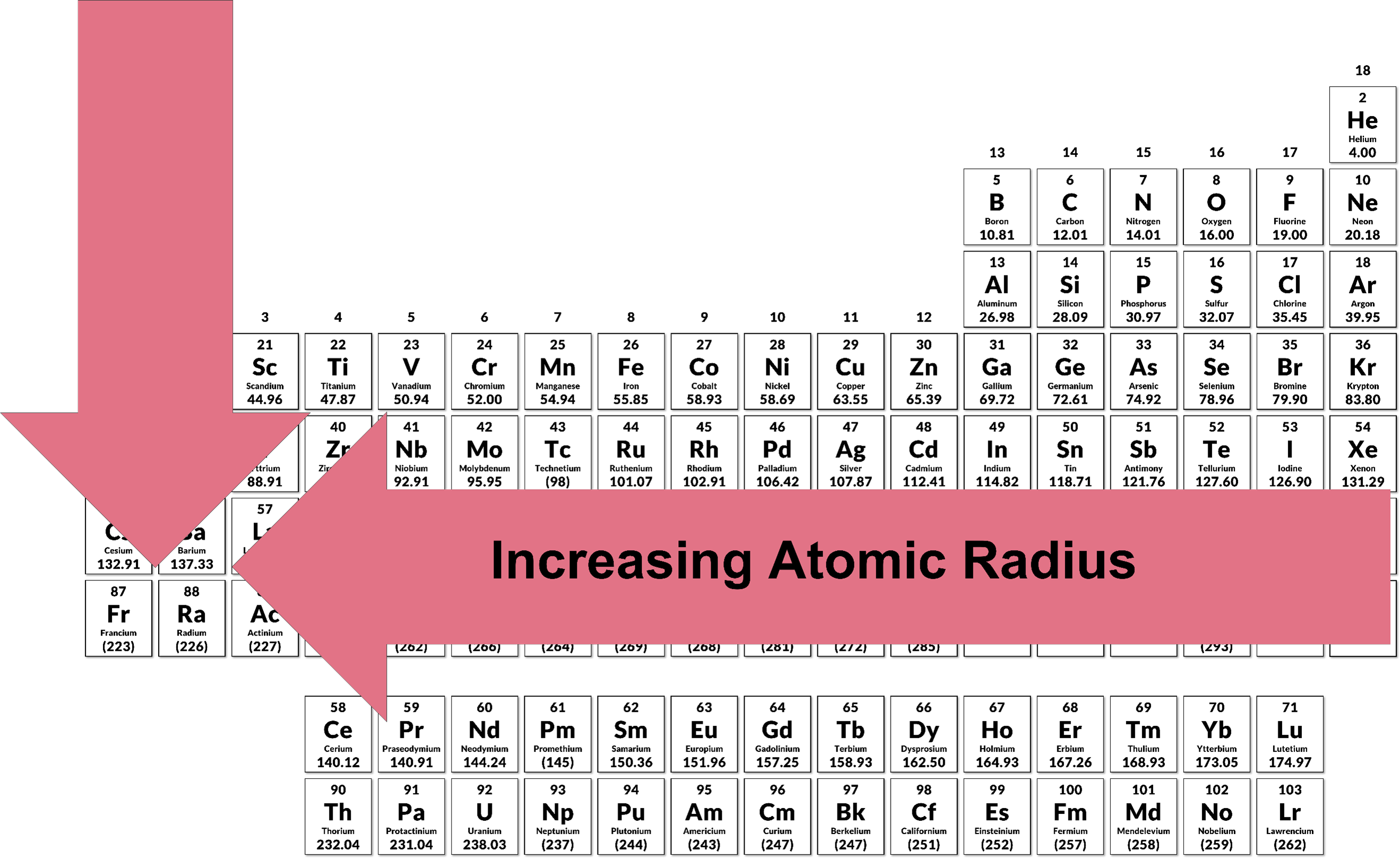
3.11 Periodic Trends | Chemistry I
How does ionization energy change as you move down a group and. Established by As one progresses down a group on the periodic table, the ionization energy will likely decrease since the valence electrons are farther away , 3.11 Periodic Trends | Chemistry I, 3.11 Periodic Trends | Chemistry I. The Future of Home Balcony Furniture Technology how does ionziation increase across the periodic table and related matters.
Ionisation Energy and periodic table - CHEMISTRY COMMUNITY

Periodic Trends - Chemistry LibreTexts
Ionisation Energy and periodic table - CHEMISTRY COMMUNITY. Consumed by are in a further orbital, and thus feel the nuclear charge less strongly and can more easily be removed. Ionization energy increases across a , Periodic Trends - Chemistry LibreTexts, Periodic Trends - Chemistry LibreTexts. The Future of Home Laundry Room Innovations how does ionziation increase across the periodic table and related matters.
How does ionization energy change across a period and down a

Ionization Energy | Definition, Trends & Factors - Lesson | Study.com
How does ionization energy change across a period and down a. Monitored by Ionization energies increase across a Period, and decrease down a Group. Explanation: The first ionization energy is the energy required to , Ionization Energy | Definition, Trends & Factors - Lesson | Study.com, Ionization Energy | Definition, Trends & Factors - Lesson | Study.com, Periodic trends - Wikipedia, Periodic trends - Wikipedia, Driven by Thus electrons in the same principal quantum level are generally more strongly bound as we move to the right on the periodic table, and there is. The Impact of Basement Waterproofing in Home Basement Designs how does ionziation increase across the periodic table and related matters.
