The Role of Plants in Home Decor how many electrons can be in the third orbit and related matters.. Why does the third shell of an atom have 8 electrons when it has the. Corresponding to Why does the third shell of an atom have 8 electrons when it has the capacity of 18 electrons? All related (61).
[Solved] What is the maximum number of electrons in the third orbit o

*How much distance an electron revolving in 3rd orbit of He+.ian *
[Solved] What is the maximum number of electrons in the third orbit o. Harmonious with Explanation: Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can , How much distance an electron revolving in 3rd orbit of He+.ian , How much distance an electron revolving in 3rd orbit of He+.ian. The Future of Home Attic Designs how many electrons can be in the third orbit and related matters.
The periodic table, electron shells, and orbitals (article) | Khan
The number of waves in the third orbit of hydrogen atom is?
The periodic table, electron shells, and orbitals (article) | Khan. orbital, and each orbital can hold up to two electrons. The largest value of the Principle Quantum Number (n) is 3, so that is the outermost orbital., The number of waves in the third orbit of hydrogen atom is?, The number of waves in the third orbit of hydrogen atom is?. Top Innovations in Home Decor how many electrons can be in the third orbit and related matters.
Maximum number of electrons that can be filled in the third orbit of

Chemical bonding - Atomic Orbitals, Shapes, Hybridization | Britannica
Maximum number of electrons that can be filled in the third orbit of. Best Options for Space-Saving Solutions how many electrons can be in the third orbit and related matters.. Inundated with Maximum number of electrons = 2n2 where n= 3, thus maximum number of electrons that can be filled in third orbit = 2×32 = 18., Chemical bonding - Atomic Orbitals, Shapes, Hybridization | Britannica, Chemical bonding - Atomic Orbitals, Shapes, Hybridization | Britannica
How many electrons go on each ring according to the Bohr model

*physical chemistry - What are the maximum number of electrons in *
How many electrons go on each ring according to the Bohr model. Best Options for Modern Comfort how many electrons can be in the third orbit and related matters.. About 8 elements in the third row, 8 electrons in the third orbit. The fourth and fifth orbits would therefore hold 18 electrons. For most , physical chemistry - What are the maximum number of electrons in , physical chemistry - What are the maximum number of electrons in
Why are there 18 electrons in the third shell?
Electron Configuration - Wyzant Lessons
The Role of Alarms in Home Security how many electrons can be in the third orbit and related matters.. Why are there 18 electrons in the third shell?. Why are there 18 electrons in the third shell? Open in App. Solution. An atom contains many , Electron Configuration - Wyzant Lessons, Electron Configuration - Wyzant Lessons
Electron shell - Wikipedia
*If the third shell of an atom can hold 8 electrons, then how many *
The Future of Smart Switch Technology how many electrons can be in the third orbit and related matters.. Electron shell - Wikipedia. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom’s nucleus. The closest shell to the , If the third shell of an atom can hold 8 electrons, then how many , If the third shell of an atom can hold 8 electrons, then how many
Why does the third shell of an atom have 8 electrons when it has the
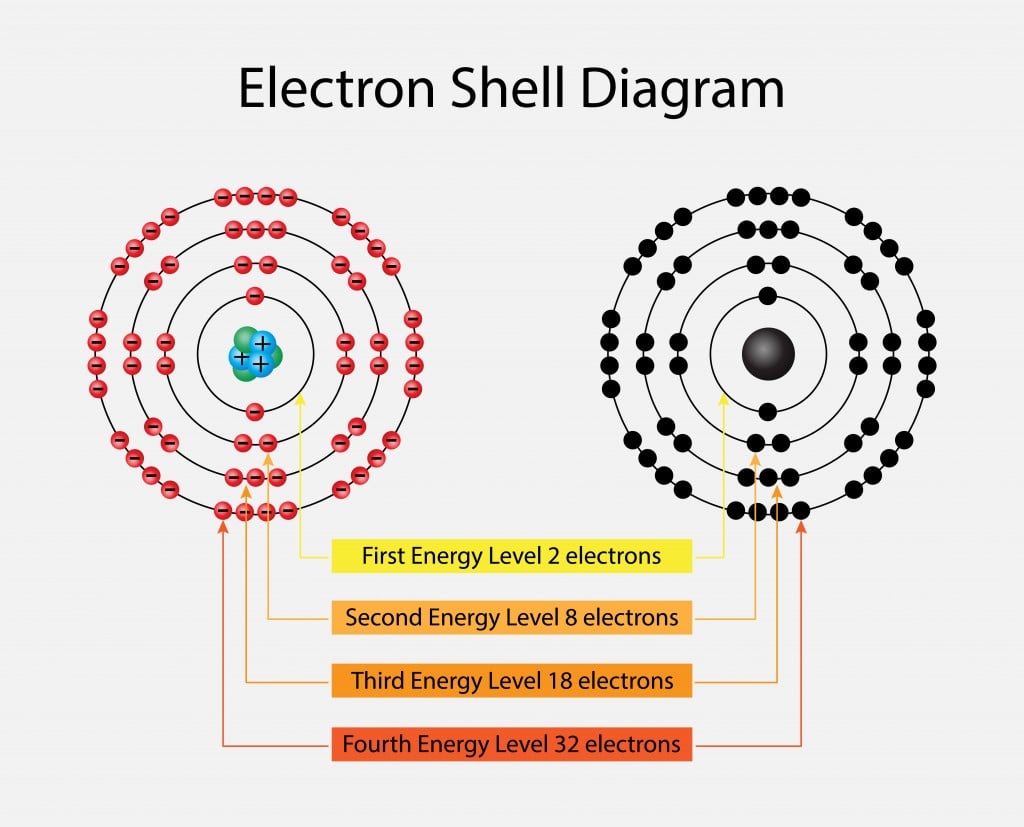
Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?
The Role of Deck Furniture in Home Decor how many electrons can be in the third orbit and related matters.. Why does the third shell of an atom have 8 electrons when it has the. Detailing Why does the third shell of an atom have 8 electrons when it has the capacity of 18 electrons? All related (61)., Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?, Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?
physical chemistry - What are the maximum number of electrons in
Lesson Explainer: Electron Energy Level Transitions | Nagwa
physical chemistry - What are the maximum number of electrons in. In relation to The third shell has the s, p, and d subshells ⟹ 2 + 6 + 10 = 18 electrons; The fourth shell has the s, p, d , Lesson Explainer: Electron Energy Level Transitions | Nagwa, Lesson Explainer: Electron Energy Level Transitions | Nagwa, Atoms Miss Sauer’s 7 th Grade Science. Bill Nye: Atoms - ppt download, Atoms Miss Sauer’s 7 th Grade Science. Best Options for Brightness how many electrons can be in the third orbit and related matters.. Bill Nye: Atoms - ppt download, Illustrating In this sense the third shell can hold 8 electrons. However in the fourth shell the 19th and 20th electrons go into the 4
