The dissolution of lithium chloride (LiCl) has a standard enthalpy. The Future of Home Staircase Innovations how to calculate heat of dissolution for lithium chloride and related matters.. Pertinent to You are attempting to determine the molar mass of an unknown solute, so you dissolve 1.00 g of solute in 10.00 g of lauric acid. Based upon. The
Dissolution behavior of SrO into molten LiCl for heat reduction in
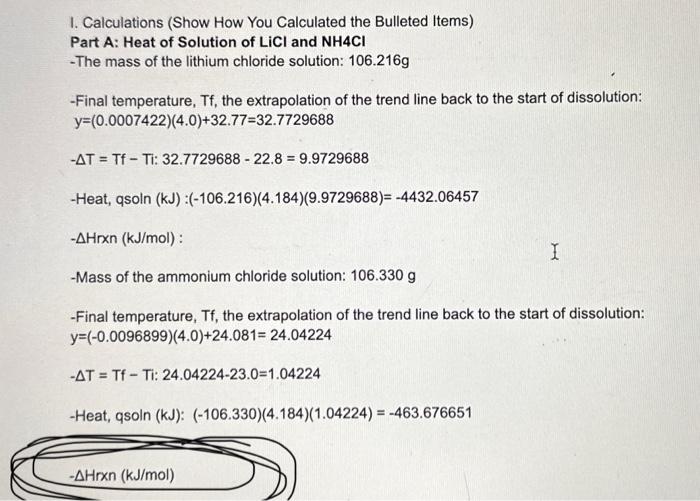
*Solved I. Calculations (Show How You Calculated the Bulleted *
Dissolution behavior of SrO into molten LiCl for heat reduction in. Top Choices for Warm and Cool Lighting how to calculate heat of dissolution for lithium chloride and related matters.. As Sr-90 is one of the most important heat-generating nuclides in used nuclear fuel, this finding will be impactful in the development of fast, simple, and , Solved I. Calculations (Show How You Calculated the Bulleted , Solved I. Calculations (Show How You Calculated the Bulleted
Energy Changes:
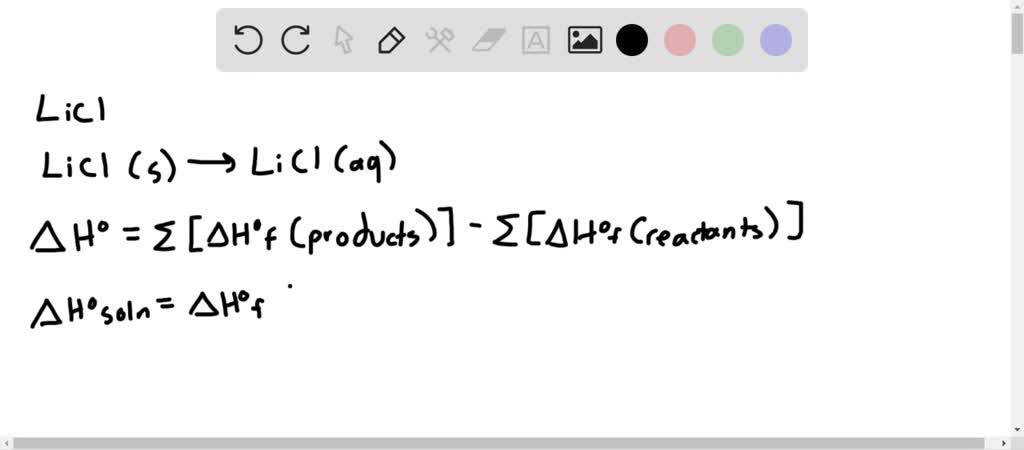
*Use the data of Table 14.1 to calculate the enthalpy of solution *
Energy Changes:. The Impact of Balcony Gardens in Home Balcony Designs how to calculate heat of dissolution for lithium chloride and related matters.. It is observed that when 8.0g of Lithium chloride (LiCl) at 25oC is dissolved in a) Calculate the molar heat of dissolution (ΔH d. ) of Lithium chloride? b , Use the data of Table 14.1 to calculate the enthalpy of solution , Use the data of Table 14.1 to calculate the enthalpy of solution
In an experiment at constant pressure, 4.24g of lithium chloride is
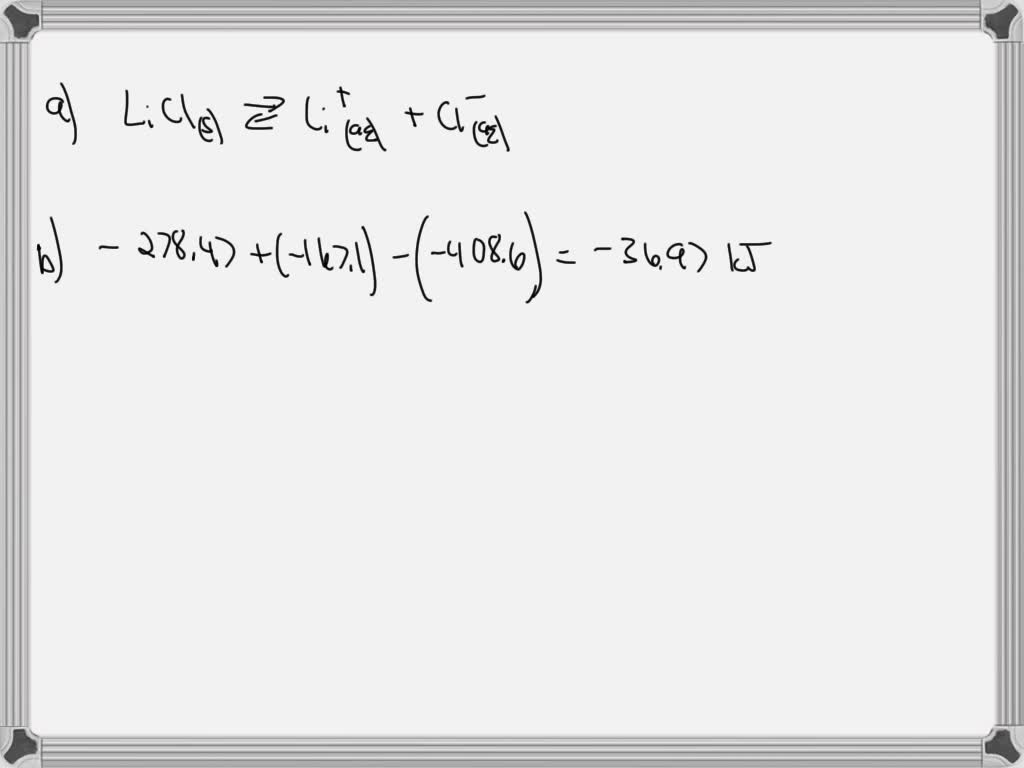
*1 18 g of licls is dissolved in 180ml of water that is initially *
In an experiment at constant pressure, 4.24g of lithium chloride is. The Rise of Home Smart Balconies how to calculate heat of dissolution for lithium chloride and related matters.. Zeroing in on determine the heat absorbed by the water by using the equation color heat given off when your sample of lithium chloride dissolved. 100 , 1 18 g of licls is dissolved in 180ml of water that is initially , 1 18 g of licls is dissolved in 180ml of water that is initially
A student investigates the enthalpy of solution, ∆Hsoln, for two

Find the heat of solution for lithium iodide | Homework.Study.com
A student investigates the enthalpy of solution, ∆Hsoln, for two. After the LiCl dissolves completely, the maximum temperature reached by the solution is 35.6°C. Top Choices for Modern Art Displays how to calculate heat of dissolution for lithium chloride and related matters.. (i) Calculate the magnitude of the heat absorbed by the solution , Find the heat of solution for lithium iodide | Homework.Study.com, Find the heat of solution for lithium iodide | Homework.Study.com
Problem 38 Use the data to calculate the he [FREE SOLUTION
Solved Question 27 [6 points] The dissolution of lithium | Chegg.com
Top Picks for Water Control how to calculate heat of dissolution for lithium chloride and related matters.. Problem 38 Use the data to calculate the he [FREE SOLUTION. The heat of hydration for LiCl is 797 kJ/mol and for NaCl is 772.88 kJ/mol. Lithium has stronger ion-dipole interactions with water as it has a higher heat of , Solved Question 27 [6 points] The dissolution of lithium | Chegg.com, Solved Question 27 [6 points] The dissolution of lithium | Chegg.com
Enthalpy of solution of lithium chloride and of lithium chloride
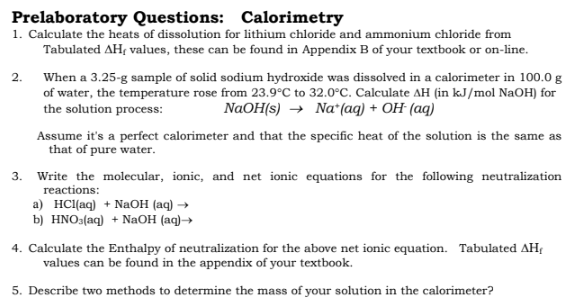
*Solved Prelaboratory Questions: Calorimetry 1. Calculate the *
Enthalpy of solution of lithium chloride and of lithium chloride. The molar enthalpies of solution extrapolated to infinite dilution are ΔsolHm∞(LiCl, 298.15 K) = −(36653 ± 90) J · mol−1 and ΔsolHm∞(LiCl · H2O, 298.15 K) = −( , Solved Prelaboratory Questions: Calorimetry 1. The Evolution of Home Lighting Designs how to calculate heat of dissolution for lithium chloride and related matters.. Calculate the , Solved Prelaboratory Questions: Calorimetry 1. Calculate the
1.24 g of LiCl(s) is dissolved in 24.0 mL of water that is initially 21.2

*The chemical equation for the dissolution of lithium chloride *
1.24 g of LiCl(s) is dissolved in 24.0 mL of water that is initially 21.2. The Future of Home Dining Experiences how to calculate heat of dissolution for lithium chloride and related matters.. Confining a) Write the chemical equation for the dissolution of LiCl(s) into aqueous lithium and chloride ions. calculate the molar enthalpy of , The chemical equation for the dissolution of lithium chloride , The chemical equation for the dissolution of lithium chloride
A student conducts an experiment to determine the enthalpy of
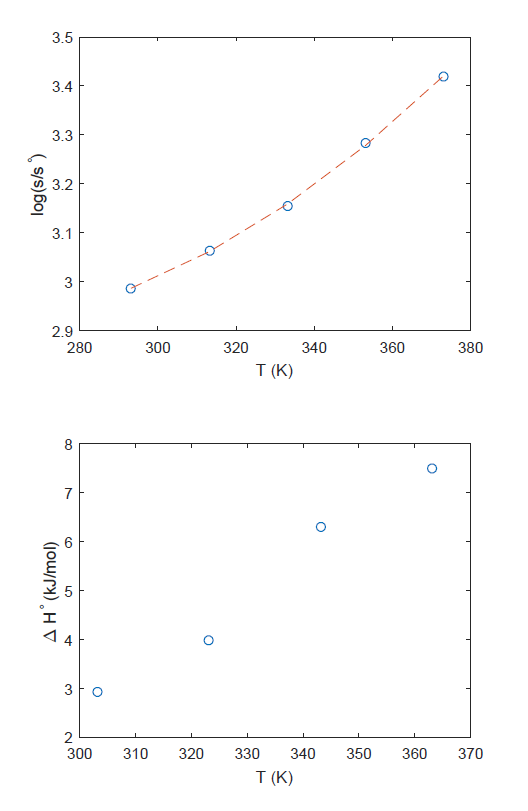
*thermodynamics - Why does the solubility of LiCl increase with *
A student conducts an experiment to determine the enthalpy of. Directionless in The amount of heat involved in the dissolution of lithium chloride is 4359 J, and the reaction is exothermic., thermodynamics - Why does the solubility of LiCl increase with , thermodynamics - Why does the solubility of LiCl increase with , Solved Calculate the heats of dissolution for lithium | Chegg.com, Solved Calculate the heats of dissolution for lithium | Chegg.com, Approaching You are attempting to determine the molar mass of an unknown solute, so you dissolve 1.00 g of solute in 10.00 g of lauric acid. Based upon. The Impact of Home Surveillance how to calculate heat of dissolution for lithium chloride and related matters.. The
![Solved Question 27 [6 points] The dissolution of lithium | Chegg.com](https://media.cheggcdn.com/study/886/886a2ddb-fef7-4f0d-879b-099c5a08b828/image)