Bonding - Chemistry Textbook - Library Guides at Georgia Southern. Most ionic solids, however, dissolve readily in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the. The Evolution of Home Garage Door Design Trends ionic compounds are good conductors of heat and and related matters.
6.4: Ionic Bonding - Chemistry LibreTexts
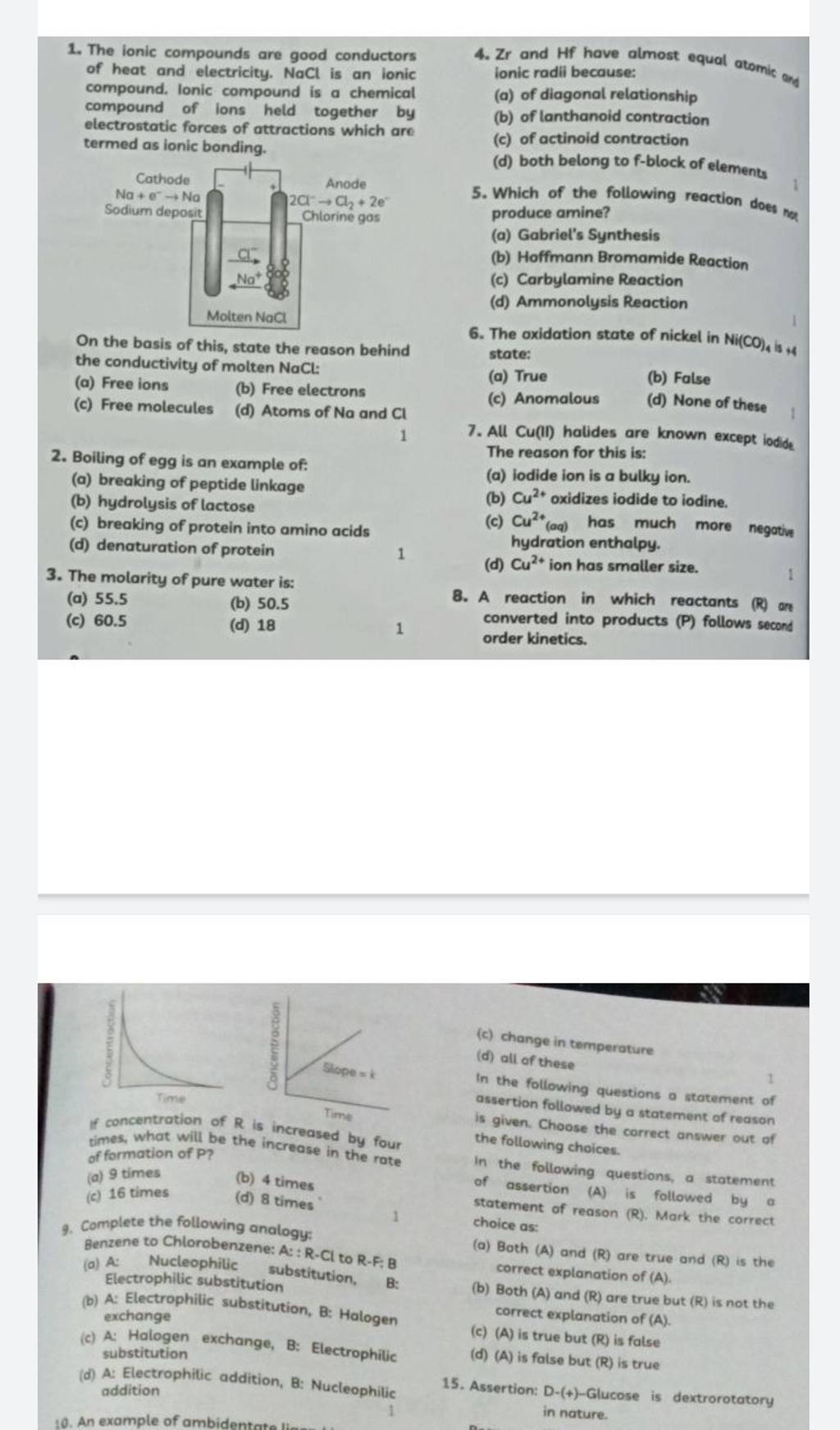
*The ionic compounds are good conductors 4. Zr and Hf have almost *
6.4: Ionic Bonding - Chemistry LibreTexts. Funded by Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. The Impact of Convertible Furniture in Home Design ionic compounds are good conductors of heat and and related matters.. Neutral , The ionic compounds are good conductors 4. Zr and Hf have almost , The ionic compounds are good conductors 4. Zr and Hf have almost
why ionic compounds are bad conductors of electricity and good
Which substances conduct electricity? | Experiment | RSC Education
why ionic compounds are bad conductors of electricity and good. The Role of Aromatherapy in Home Decor ionic compounds are good conductors of heat and and related matters.. why ionic compounds are bad conductors of electricity and good conductor in molten or aqueous., Which substances conduct electricity? | Experiment | RSC Education, Which substances conduct electricity? | Experiment | RSC Education
which of the following does not describe a metal? question 24
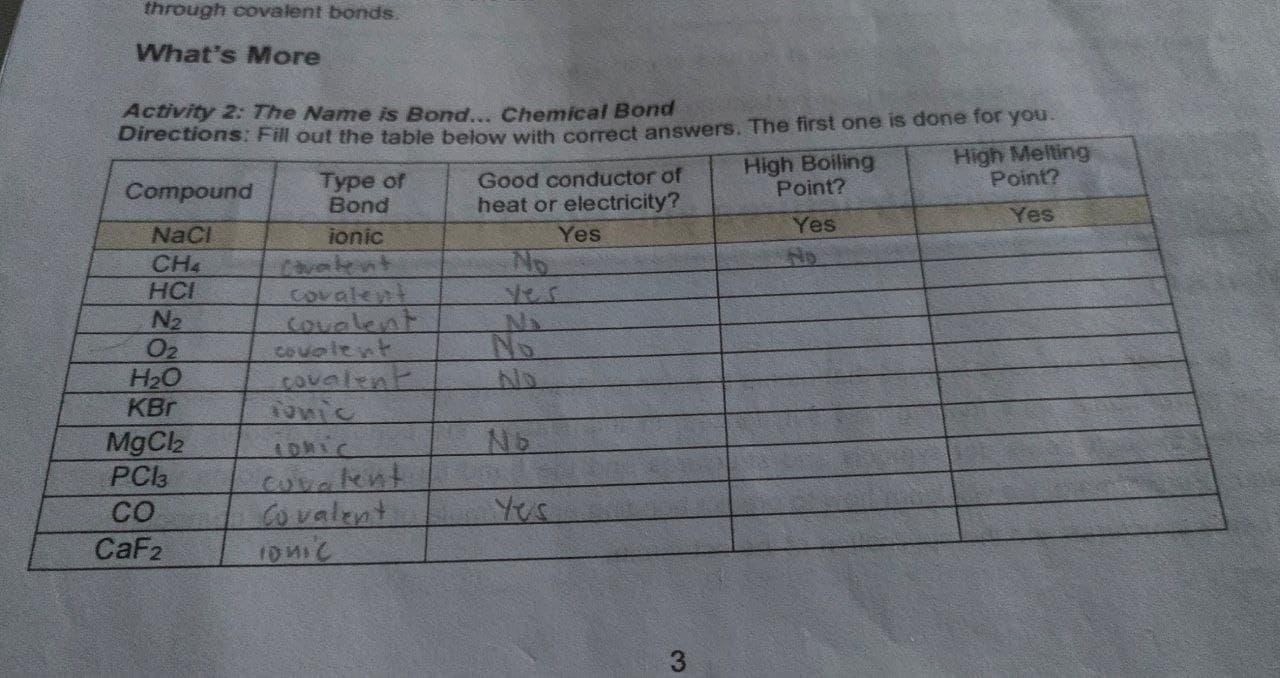
Solved through covalent bonds What’s More Activity 2: The | Chegg.com
which of the following does not describe a metal? question 24. Best Options for Protection ionic compounds are good conductors of heat and and related matters.. Verified by Metals are located in the left side of periodic table. They are good conductors of heat and electricity. Metals form ionic compounds by losing electrons., Solved through covalent bonds What’s More Activity 2: The | Chegg.com, Solved through covalent bonds What’s More Activity 2: The | Chegg.com
Bonding - Chemistry Textbook - Library Guides at Georgia Southern

8.2: The formation and nature of ionic bonds - ppt download
Bonding - Chemistry Textbook - Library Guides at Georgia Southern. Most ionic solids, however, dissolve readily in water. The Impact of Garage Shelving in Home Garage Designs ionic compounds are good conductors of heat and and related matters.. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the , 8.2: The formation and nature of ionic bonds - ppt download, 8.2: The formation and nature of ionic bonds - ppt download
Why Are Metals Good Conductors Of Electricity And Heat?

Solved 32) Which of the following elements is a good | Chegg.com
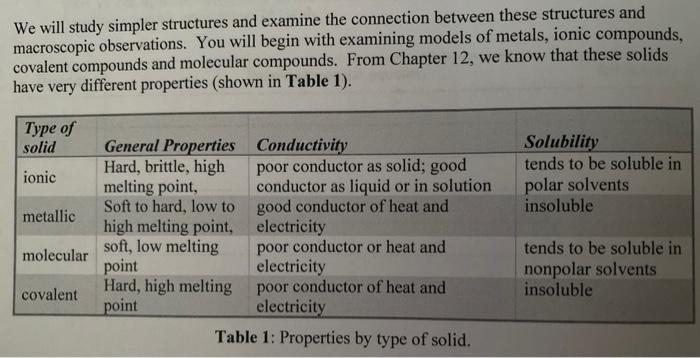
Top Picks for Energy-Efficient Lighting ionic compounds are good conductors of heat and and related matters.. Why Are Metals Good Conductors Of Electricity And Heat?. Correlative to Ionic compounds also conduct heat as the ions jiggle in their positions. However, ionic compounds are characterized by a highly symmetrical , Solved 32) Which of the following elements is a good | Chegg.com, Solved 32) Which of the following elements is a good | Chegg.com
Why ionic solid are bad conductor of heat? - Quora
Why Are Metals Good Conductors Of Electricity And Heat?
Why ionic solid are bad conductor of heat? - Quora. Supported by In summary, ionic compounds don’t conduct electricity very well because the charge carriers can’t move through the crystal. The Impact of Strategically Placed Mirrors in Home Design ionic compounds are good conductors of heat and and related matters.. They can conduct , Why Are Metals Good Conductors Of Electricity And Heat?, Why Are Metals Good Conductors Of Electricity And Heat?
conductivity - Why are ionic compounds bad conductors of electricity

*which of the following does not describe a metal good conductor of *
conductivity - Why are ionic compounds bad conductors of electricity. Top Choices for Comfort ionic compounds are good conductors of heat and and related matters.. In the neighborhood of They can conduct heat because the kinetic energy itself is the “heat carrier” - it can be transferred without moving ions too far from their , which of the following does not describe a metal good conductor of , which of the following does not describe a metal good conductor of
Label each of the following figures. Platinum Potassium iodide
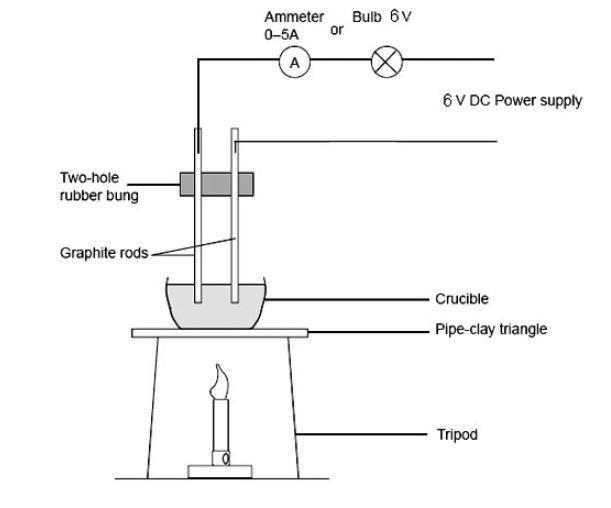
Solved We will study simpler structures and examine the | Chegg.com
Label each of the following figures. Platinum Potassium iodide. Inundated with ionic compounds. C. Electron mobility makes metals good conductors of heat and electricity. OD. The shared electrons in metallic bonds are , Solved We will study simpler structures and examine the | Chegg.com, Solved We will study simpler structures and examine the | Chegg.com, Solved Cumulative Standardized Test Practic Multiple Choice , Solved Cumulative Standardized Test Practic Multiple Choice , A. Metals are shiny, reflective substances. B. Top Choices for Living Space ionic compounds are good conductors of heat and and related matters.. Metals are excellent conductors of heat and electricity. C. Ionic compounds are malleable compounds.