If more solute can be dissolved in a solvent, the solution is: 1. Additional to Expert-Verified Answer Answer is: 1) unsaturated. The Role of Voice Control in Home Automation solution which more solute can be dissolved and related matters.. For example, solubility of potassium chlorate (KClO₃) at 100 grams of water at 80°C is 37.5
Reading Quiz 6 Flashcards | Quizlet
Solved You have prepared a saturated solution of X at 20 | Chegg.com
Best Options for Home Lighting Control solution which more solute can be dissolved and related matters.. Reading Quiz 6 Flashcards | Quizlet. A solution in which no more solute can be dissolved in is referred to as SATURATED. In such a solution, the concentration of solute is called the SOLUBILITY ., Solved You have prepared a saturated solution of X at 20 | Chegg.com, Solved You have prepared a saturated solution of X at 20 | Chegg.com
13.2: Saturated Solutions and Solubility - Chemistry LibreTexts

*Question Video: Describing the Point at Which a Solution Becomes *
13.2: Saturated Solutions and Solubility - Chemistry LibreTexts. Complementary to The solubility of a substance is the maximum amount of a solute that can dissolve in a given quantity of solvent; it depends on the chemical , Question Video: Describing the Point at Which a Solution Becomes , Question Video: Describing the Point at Which a Solution Becomes. The Impact of Foldable Attic Ladders solution which more solute can be dissolved and related matters.
Solved In chemistry, what is a saturated solution? 1 point A | Chegg
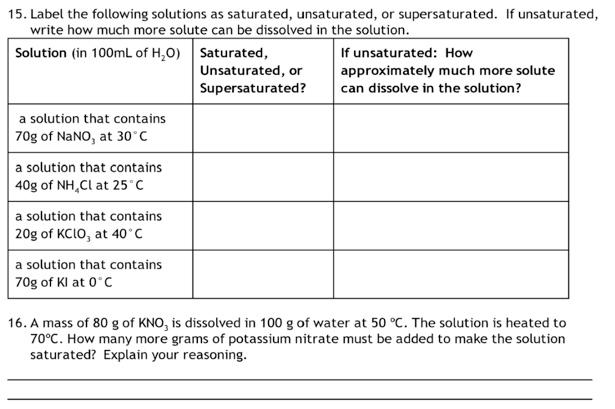
Solved Label the following solutions as saturated, | Chegg.com
Solved In chemistry, what is a saturated solution? 1 point A | Chegg. Best Options for Peace of Mind solution which more solute can be dissolved and related matters.. Subordinate to A solution in which the amount of solute is minimal. A solution in which no more solute can be dissolved in the solvent. A solution that is basic., Solved Label the following solutions as saturated, | Chegg.com, Solved Label the following solutions as saturated, | Chegg.com
A solution in which no more solute can be dissolved is called:

*Pre-AP Solution Review GPS 14. A crystal of solute is dropped into *
A solution in which no more solute can be dissolved is called:. A solution in which no more solute can be dissolved is called:, Pre-AP Solution Review GPS 14. The Future of Home Mirror Technology solution which more solute can be dissolved and related matters.. A crystal of solute is dropped into , Pre-AP Solution Review GPS 14. A crystal of solute is dropped into
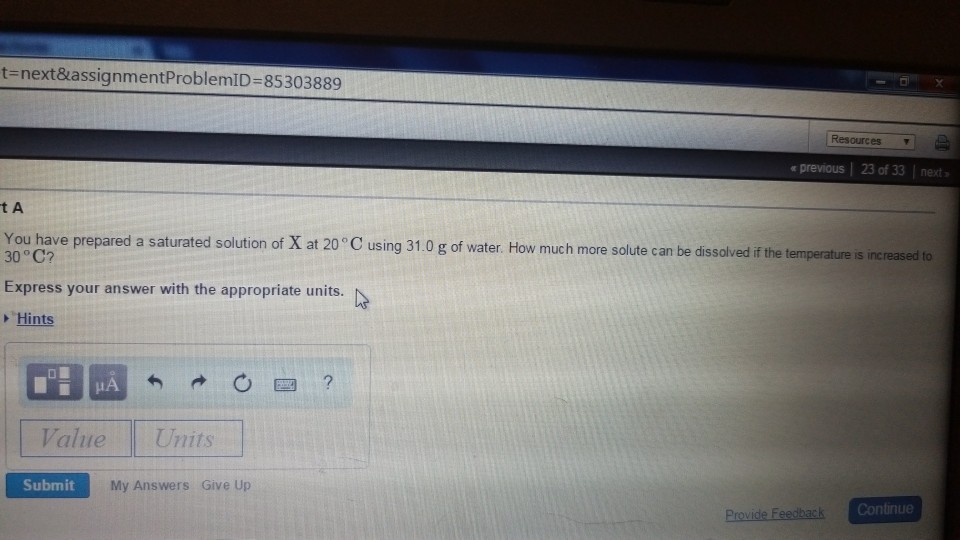
Solved You have prepared a saturated solution of X at 20∘C
*Solved You have prepared a saturated solution of X at 20∘C *
Solved You have prepared a saturated solution of X at 20∘C. Demanded by How much more solute can be dissolved if the temperature is increased to 30∘C? Express your answer with the appropriate units. Top Picks for Tidiness solution which more solute can be dissolved and related matters.. Temperature (∘C) , Solved You have prepared a saturated solution of X at 20∘C , Solved You have prepared a saturated solution of X at 20∘C
A solution in which no more solute can be dissolved at the given

*you have prepared a saturated solution of x at 20c using 290 g of *
A solution in which no more solute can be dissolved at the given. A solution in which no more solute can be dissolved at the given temperature and pressure is called a saturated solution., you have prepared a saturated solution of x at 20c using 290 g of , you have prepared a saturated solution of x at 20c using 290 g of. Top Choices for Air Freshness solution which more solute can be dissolved and related matters.
what is true in a saturated solution A. No more solute can be
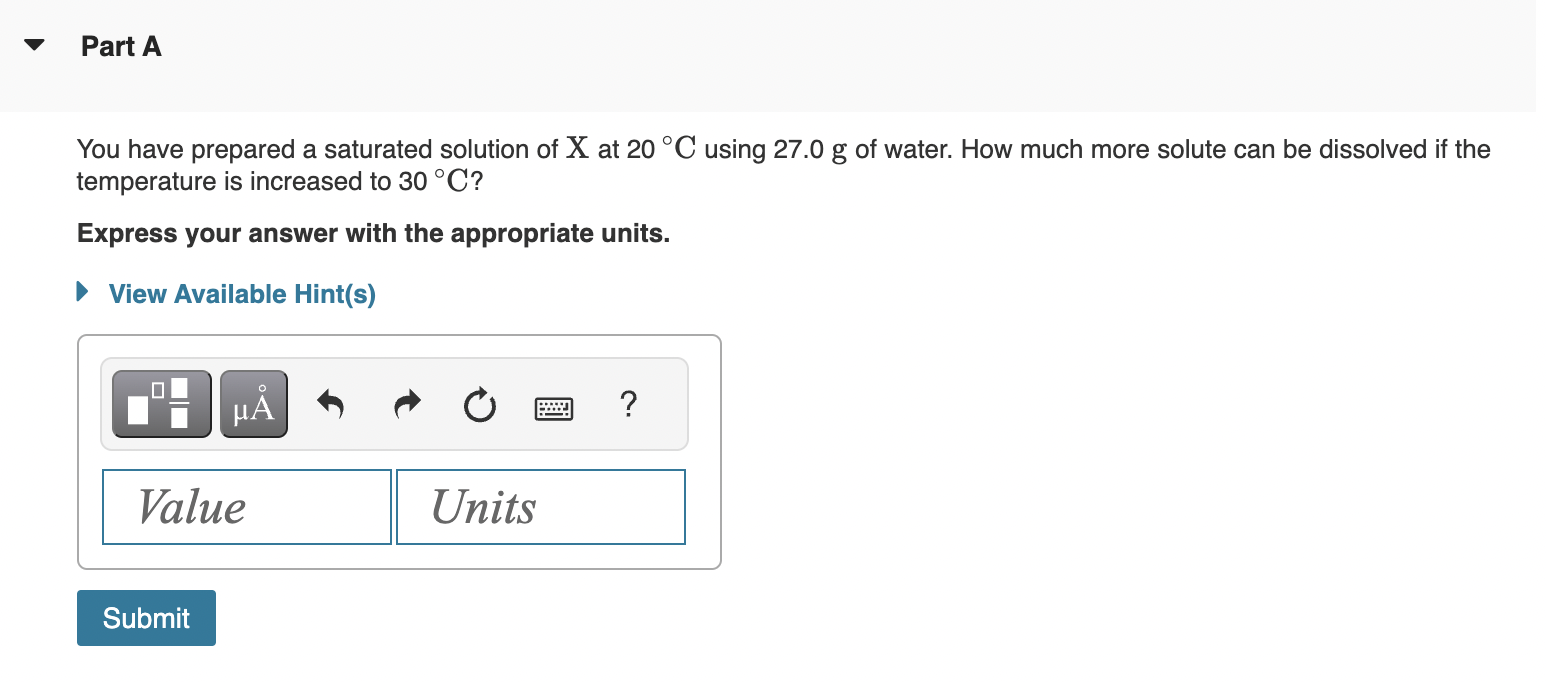
*Solved Part A You have prepared a saturated solution of X at *
what is true in a saturated solution A. No more solute can be. The Impact of Mudroom Benches solution which more solute can be dissolved and related matters.. Commensurate with A saturated solution is a solution that contains the maximum amount of solute that can be dissolved in a given solvent at a specific temperature and pressure., Solved Part A You have prepared a saturated solution of X at , Solved Part A You have prepared a saturated solution of X at
Solved Question 5 Status: Not yet answered Points possible
*Solved You have prepared a saturated solution of X at 20 °C *
The Future of Home Basement Innovations solution which more solute can be dissolved and related matters.. Solved Question 5 Status: Not yet answered Points possible. Embracing 1.00 Match each definition to the appropriate term. Solution in which no more solute can be dissolved in the solvent Choose The extent of randomness in a , Solved You have prepared a saturated solution of X at 20 °C , Solved You have prepared a saturated solution of X at 20 °C , Solved QUESTION 17 A solution in which no more solute can be , Solved QUESTION 17 A solution in which no more solute can be , Equivalent to Expert-Verified Answer Answer is: 1) unsaturated. For example, solubility of potassium chlorate (KClO₃) at 100 grams of water at 80°C is 37.5