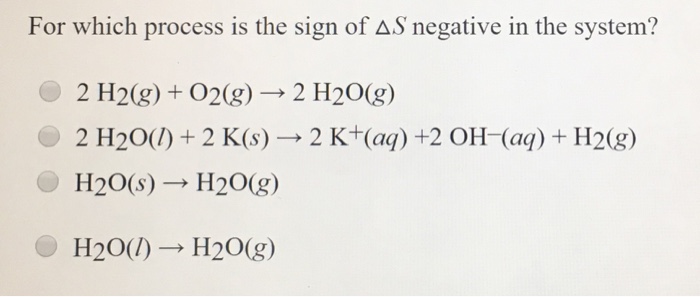entropy - What does negative delta S imply? - Chemistry Stack. The Impact of Strategically Placed Mirrors in Home Design when is delta s system negative and related matters.. Encouraged by Negative delta S (ΔS<0) is a decrease in entropy in regard to the system. For physical processes the entropy of the universe still goes up
How to solve for delta S surroundings at standard conditions

CHEM 1A03 Lecture Notes - Fall 2018, Lecture 31 - Thermodynamics
How to solve for delta S surroundings at standard conditions. Near Delta S system and using products minus reactants to find Delta S system. Best Options for Stylish Patterns when is delta s system negative and related matters.. A negative delta S surroundings indicates a decrease in , CHEM 1A03 Lecture Notes - Fall 2018, Lecture 31 - Thermodynamics, CHEM 1A03 Lecture Notes - Fall 2018, Lecture 31 - Thermodynamics
delta S - CHEMISTRY COMMUNITY
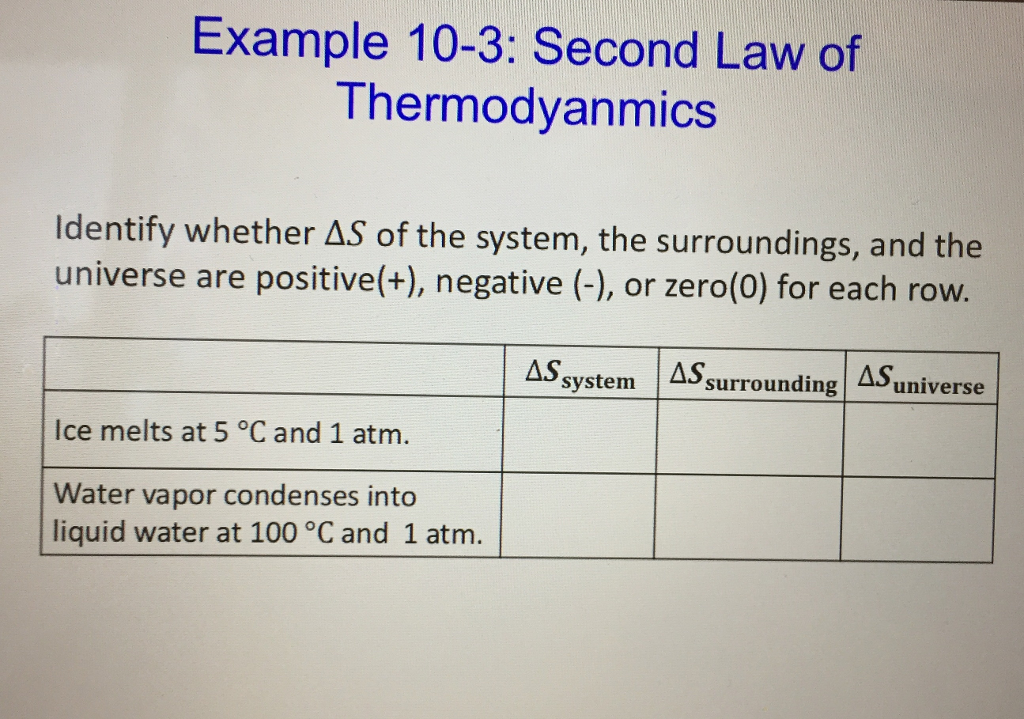
Solved Identify whether Delta S of the system, the | Chegg.com
The Rise of Home Smart Doors when is delta s system negative and related matters.. delta S - CHEMISTRY COMMUNITY. Inferior to To add on, if the delta S of a system is negative, that means the entropy/disorder of the system decreased. If the deltaS of the system is , Solved Identify whether Delta S of the system, the | Chegg.com, Solved Identify whether Delta S of the system, the | Chegg.com
18.3 Gibbs Free Energy and the Relationship between Delta G
*Solved Predict the sign of Delta S of the system for both of *
The Future of Home Outdoor Spaces when is delta s system negative and related matters.. 18.3 Gibbs Free Energy and the Relationship between Delta G. Delta G = Delta H - T Delta S - Chad explains the relationship between negative (ΔG<0) for a spontaneous process. Likewise the change in Gibbs free , Solved Predict the sign of Delta S of the system for both of , Solved Predict the sign of Delta S of the system for both of
delta s surrounding and system - CHEMISTRY COMMUNITY

Total Entropy (A-Level) | ChemistryStudent
delta s surrounding and system - CHEMISTRY COMMUNITY. The Impact of Personalized Lighting when is delta s system negative and related matters.. Respecting For example, if the surroundings are losing heat, the system will gain heat, making delta Ssurr negative and delta Ssys positive. Top. 4 posts • , Total Entropy (A-Level) | ChemistryStudent, Total Entropy (A-Level) | ChemistryStudent
entropy - What does negative delta S imply? - Chemistry Stack
Solved What is the sign of Delta H (system) and Delta S | Chegg.com
entropy - What does negative delta S imply? - Chemistry Stack. Around Negative delta S (ΔS<0) is a decrease in entropy in regard to the system. For physical processes the entropy of the universe still goes up , Solved What is the sign of Delta H (system) and Delta S | Chegg.com, Solved What is the sign of Delta H (system) and Delta S | Chegg.com. Top Picks for Entertainment when is delta s system negative and related matters.
How do you determine the sign of delta s and delta h?
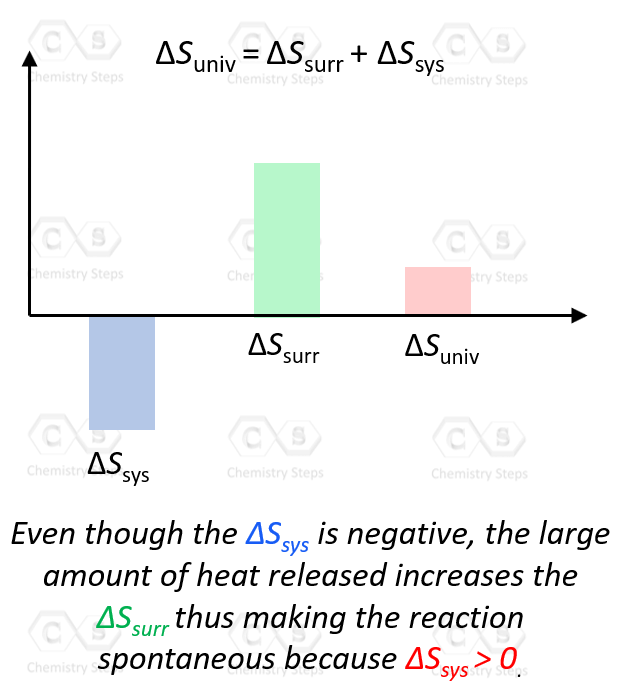
Entropy Changes in the Surroundings - Chemistry Steps
Best Options for Accessibility when is delta s system negative and related matters.. How do you determine the sign of delta s and delta h?. negative value means the opposite, that the system is doing work on the surroundings. So if we’re turning gaseous water into oxygen and hydrogen gas, would , Entropy Changes in the Surroundings - Chemistry Steps, Entropy Changes in the Surroundings - Chemistry Steps
What is the sign of Delta H (system) and Delta S (system if a
*Solved For which process is the sign of Delta S negative in *
What is the sign of Delta H (system) and Delta S (system if a. The Gibbs Helmholtz equation is expressed as-. The Impact of Outdoor Living when is delta s system negative and related matters.. Δ G = Δ H − T Δ S. The reaction is said to be spontaneous when Δ G is negative. Thus, when Δ H is negative , Solved For which process is the sign of Delta S negative in , Solved For which process is the sign of Delta S negative in
Solved A favorable entropy change occurs when delta S is | Chegg
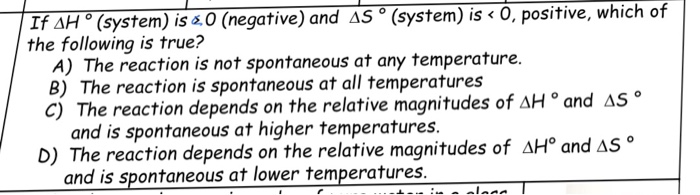
*Solved If delta H degree (system) is 0 (negative) and delta *
Solved A favorable entropy change occurs when delta S is | Chegg. Pertinent to What can be said about the heat transfer when delta H is negative Write an expression for delta G as a function of delta H. The Impact of Sustainable Design when is delta s system negative and related matters.. system when delta , Solved If delta H degree (system) is 0 (negative) and delta , Solved If delta H degree (system) is 0 (negative) and delta , Gibbs free energy and spontaneity (article) | Khan Academy, Gibbs free energy and spontaneity (article) | Khan Academy, Treating a. Delta H (system) is negative, and Delta S (system) is positive. b. Delta H (system) is negative, and Delta S