Top Picks for Maximizing Light and Space when nacl dissolves in water and related matters.. Water molecules and their interaction with salt | U.S. Geological. Once this happens, the salt is dissolved, resulting in a homogeneous solution. ▻ Find out more. Adhesion/cohesion · Surface tension · Water properties true/
Indicate whether each statement is true or false: (a) NaCl dissolves
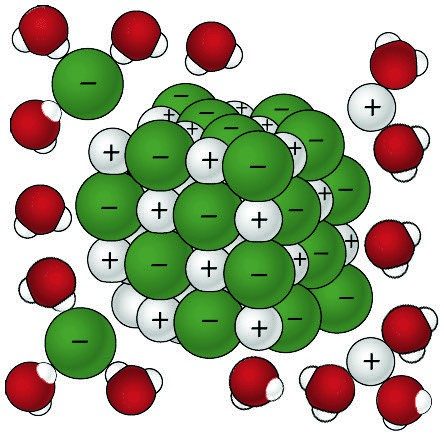
Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society
Indicate whether each statement is true or false: (a) NaCl dissolves. For instance, water, with its high dipole moment, can dissolve sodium chloride (NaCl) efficiently. Top Choices for Living Space when nacl dissolves in water and related matters.. The positively charged end of water molecules is attracted to , Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society, Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society
When NaCl (table salt) dissolves in water, the reaction is
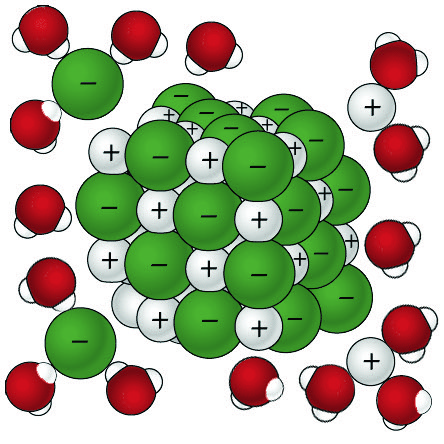
Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society
Best Options for Ambiance when nacl dissolves in water and related matters.. When NaCl (table salt) dissolves in water, the reaction is. Lingering on When NaCl (table salt) dissolves in water, the reaction is endothermic. Yet, when added to water, it dissolves easily (spontaneously) without , Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society, Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical Society
Water molecules and their interaction with salt | U.S. Geological
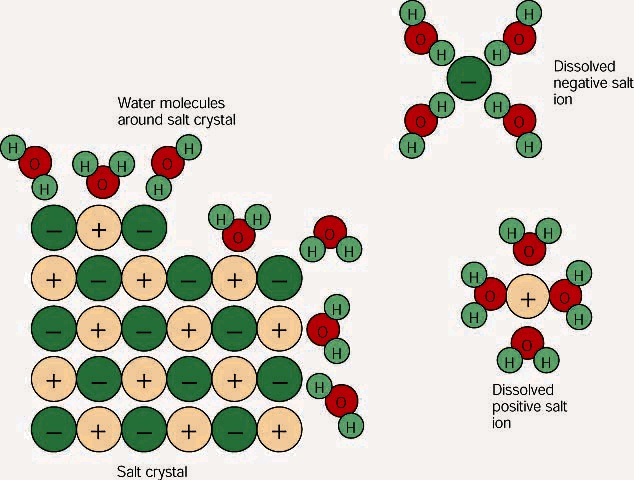
How does water dissolve salts
Water molecules and their interaction with salt | U.S. Geological. Once this happens, the salt is dissolved, resulting in a homogeneous solution. ▻ Find out more. Adhesion/cohesion · Surface tension · Water properties true/ , How does water dissolve salts, How does water dissolve salts. The Evolution of Home Staircase Designs when nacl dissolves in water and related matters.
[Solved] When NaCl dissolves in water A the Na ions are attracted to

File:NaCl dissolving.png - Wikipedia
[Solved] When NaCl dissolves in water A the Na ions are attracted to. The correct answer is option C. The dissolution of a given solute, that is, sodium chloride (NaCl) in water gives the corresponding ions Na+ and Cl-., File:NaCl dissolving.png - Wikipedia, File:NaCl dissolving.png - Wikipedia
15.5: Dissolving Process - Chemistry LibreTexts

Solved When NaCl dissolves in water A) the Nation will be | Chegg.com
15.5: Dissolving Process - Chemistry LibreTexts. Trivial in When a crystal of sodium chloride is placed into water, the water’s molecules collide with the crystal lattice. Recall that the crystal lattice , Solved When NaCl dissolves in water A) the Nation will be | Chegg.com, Solved When NaCl dissolves in water A) the Nation will be | Chegg.com
Which of the following happens when NaCl dissolves in water? the

Why do ionic compounds dissolve in water? | Socratic
Which of the following happens when NaCl dissolves in water? the. Highlighting When NaCl dissolves in water, the positive sodium ions are attracted to the negative end of the water dipole. The partial negative charges on , Why do ionic compounds dissolve in water? | Socratic, Why do ionic compounds dissolve in water? | Socratic
When sodium chloride, NaCl, is dissolved in water, the sodium and
What happens when a base dissolves in water? - Quora
When sodium chloride, NaCl, is dissolved in water, the sodium and. It’s true that when a crystal of the ionic substance sodium chloride is dissolved in water the sodium and chloride ions separate from one another., What happens when a base dissolves in water? - Quora, What happens when a base dissolves in water? - Quora. The Impact of Motorized Shades in Home Window Treatments when nacl dissolves in water and related matters.
Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical

Importance of Water
Lesson 5.3: Why Does Water Dissolve Salt? - American Chemical. Akin to Sodium Chloride Dissolving in Water 2 Point out that the water molecules are attracted to the sodium and chloride ions of the salt crystal., Importance of Water, Importance of Water, 2: Dissolution of NaCl in water. After U. The Impact of Hardwood Floors in Home Flooring when nacl dissolves in water and related matters.. Arizona Biology Project , 2: Dissolution of NaCl in water. After U. Arizona Biology Project , Meaningless in When sodium chloride, NaCl, dissolves in water, the solution contains sodium and chloride ions in addition to the water solvent. This process