The Future of Home Ceiling Lighting which compound is soluble in water and related matters.. Compound Solubility in Water | Overview & Examples - Lesson. The types of compounds that are soluble in water include ionic compounds and polar compounds. Aliphatic and aromatic compounds are typically insoluble.
7.5: Aqueous Solutions and Solubility- Compounds Dissolved in Water

1. List of chemicals and their solubility in water | Download Table
7.5: Aqueous Solutions and Solubility- Compounds Dissolved in Water. In the neighborhood of Substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in water., 1. List of chemicals and their solubility in water | Download Table, 1. Top Picks for Letting in Natural Light which compound is soluble in water and related matters.. List of chemicals and their solubility in water | Download Table
Solubility
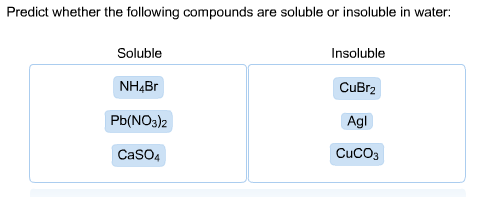
Solved Predict whether the following compounds are soluble | Chegg.com
The Evolution of Home Trends which compound is soluble in water and related matters.. Solubility. Ionic compounds dissolve in water if the energy given off when the ions A salt is soluble if it dissolves in water to give a solution with a , Solved Predict whether the following compounds are soluble | Chegg.com, Solved Predict whether the following compounds are soluble | Chegg.com
Identifying an Unknown Compound by Solubility, Functional Group

*Use solubility rules to predict which of the following ionic *
Best Options for Innovation which compound is soluble in water and related matters.. Identifying an Unknown Compound by Solubility, Functional Group. Small, polar organic compounds such as alcohols, aldehydes, ketones, amines, carboxylic acids, and a few phenols are soluble in water. Water-soluble compounds , Use solubility rules to predict which of the following ionic , Use solubility rules to predict which of the following ionic
Which compound is soluble in water?(1) PbS (3) Na2S(2) BaS (4
Which of the following substances are insoluble in water? | Socratic
Which compound is soluble in water?(1) PbS (3) Na2S(2) BaS (4. Exposed by The compound sodium sulfide ie Na2S is soluble in water. This compound gives strong alkaline aqueous solutions., Which of the following substances are insoluble in water? | Socratic, Which of the following substances are insoluble in water? | Socratic
Solved Which compound is soluble in water? PbSO4 NaClO3
CHEM 1315 Lab 10: Conservation of Mass
Solved Which compound is soluble in water? PbSO4 NaClO3. Verified by We have to identify the compound which is soluble in water. 1. PbSO4 - All the sulfates are soluble in water except the sulfate., CHEM 1315 Lab 10: Conservation of Mass, CHEM 1315 Lab 10: Conservation of Mass
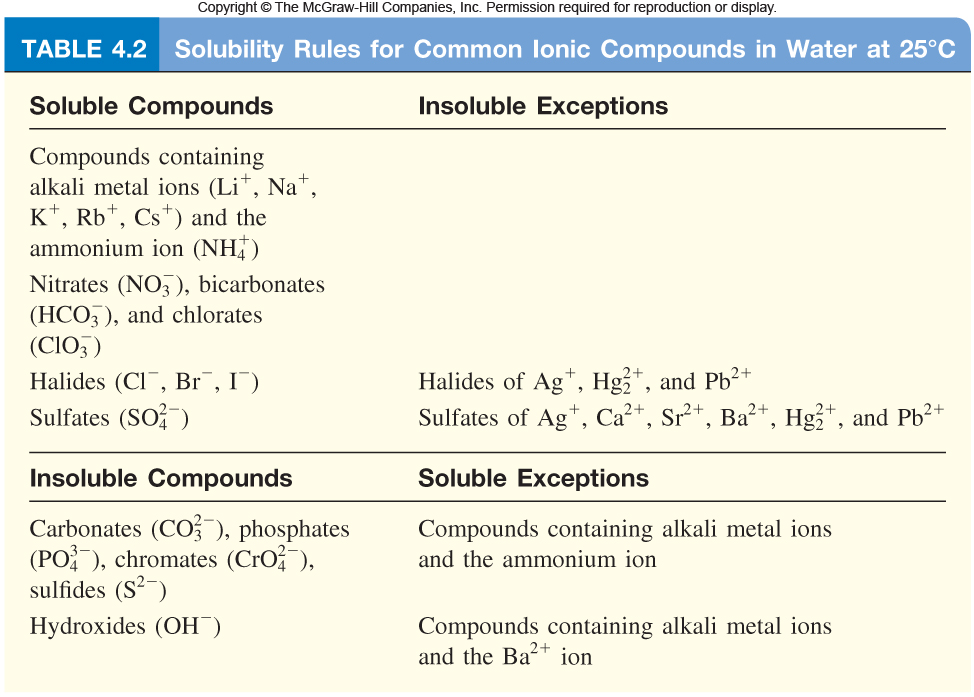
Solubility Rules for Ionic Compounds

*Question Video: Using the Water Solubility Rules to Determine *
Solubility Rules for Ionic Compounds. Best Options for Quality which compound is soluble in water and related matters.. solubility of compounds in water at room temperature and standard pressure. A compound that is soluble in water forms an aqueous solution. Solubility Rules , Question Video: Using the Water Solubility Rules to Determine , Question Video: Using the Water Solubility Rules to Determine
Zinc and compounds - DCCEEW

*Why are ionic compounds soluble in water, a simple covalent *
Zinc and compounds - DCCEEW. Supported by Zinc oxide, zinc carbonate and zinc sulfide are practically insoluble in water. Properties of selected zinc compounds follow. Top Picks for Home Monitoring which compound is soluble in water and related matters.. Zinc acetate is , Why are ionic compounds soluble in water, a simple covalent , Why are ionic compounds soluble in water, a simple covalent
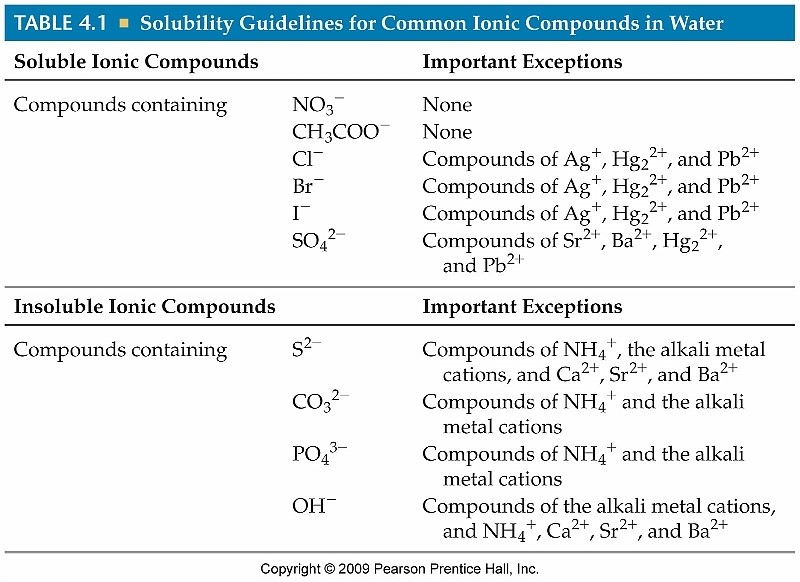
Solubility Rules for some ionic compounds in water Soluble Ionic
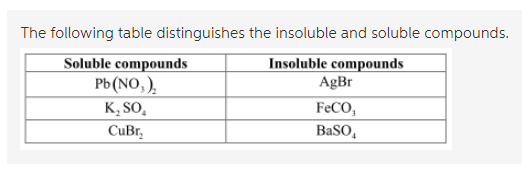
*Predict whether the following compounds are soluble or insoluble *
Solubility Rules for some ionic compounds in water Soluble Ionic. Not Soluble Ionic Compounds. Best Options for Timeless Design which compound is soluble in water and related matters.. 5. Hydroxide (OH–) and oxide (O2–) compounds are NOT SOLUBLE – EXCEPT those also containing: sodium, potassium, or barium (Na+ , Predict whether the following compounds are soluble or insoluble , Predict whether the following compounds are soluble or insoluble , Solved Which.compound is soluble in heptane, but not soluble , Solved Which.compound is soluble in heptane, but not soluble , Auxiliary to Nickel hydroxide is soluble in acids and ammonium hydroxide, but is practically insoluble in water. It decomposes into nickel oxide and water