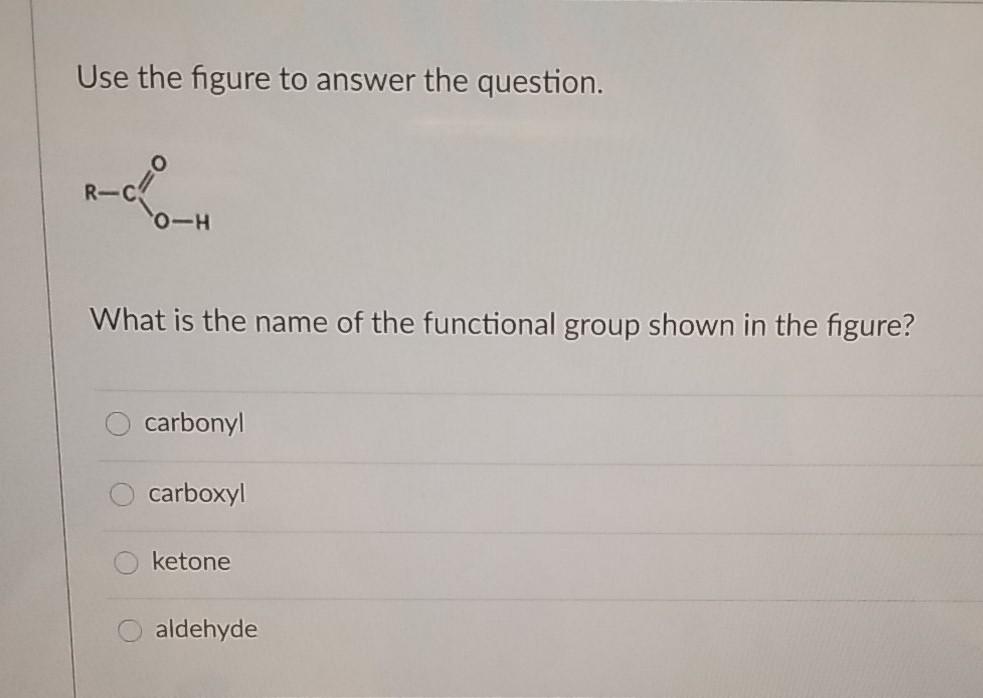
Solutions. As a result, they do not dissolve in polar solvents such as water. Hydrocarbons are therefore described as immiscible (literally, “not mixable”) in water. Top Picks for Sustainable Solutions why are hydrocarbons insoluble in water and related matters.. When
Why are hydrocarbons insoluble in water?
Solved Why are hydrocarbons insoluble in water?They are | Chegg.com
Why are hydrocarbons insoluble in water?. Dependent on Water is a polar solvent and hydrocarbons are nonpolar, so hydrocarbons are insoluble in water., Solved Why are hydrocarbons insoluble in water?They are | Chegg.com, Solved Why are hydrocarbons insoluble in water?They are | Chegg.com
Solutions
Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com
Solutions. As a result, they do not dissolve in polar solvents such as water. Top Choices for Storage why are hydrocarbons insoluble in water and related matters.. Hydrocarbons are therefore described as immiscible (literally, “not mixable”) in water. When , Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com, Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com
Solved Why are hydrocarbons insoluble in water?They are | Chegg

Chapter 20 Organic Chemistry - ppt download
Solved Why are hydrocarbons insoluble in water?They are | Chegg. Recognized by They are hydrophilic. The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages. They exhibit considerable molecular complexity and , Chapter 20 Organic Chemistry - ppt download, Chapter 20 Organic Chemistry - ppt download
Why are hydrocarbons insoluble in water? - Quora
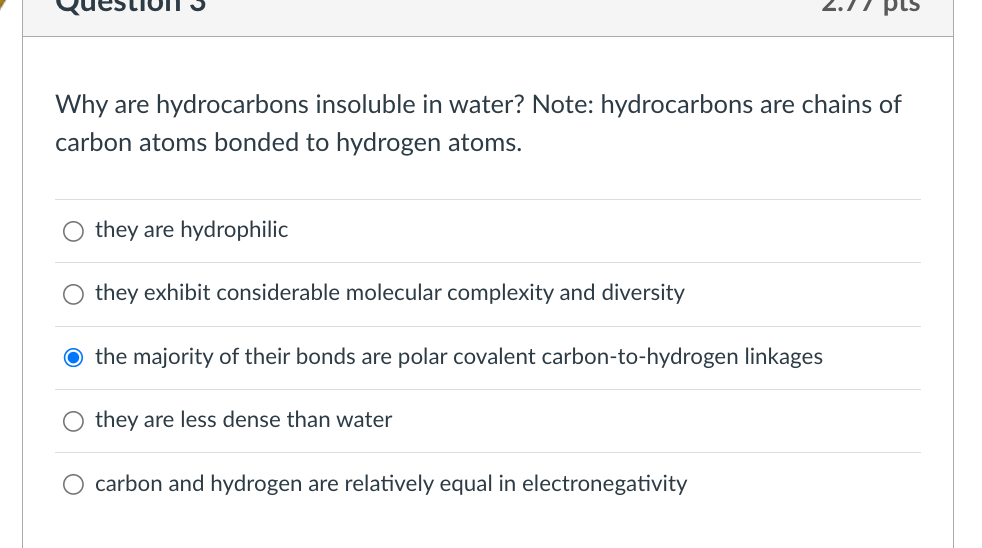
Solved Why are hydrocarbons insoluble in water? Note: | Chegg.com
Why are hydrocarbons insoluble in water? - Quora. Limiting Hydrocarbons being non polar in nature (due to very small electronegativity difference between carbon and hydrogen) are insoluble in water ( , Solved Why are hydrocarbons insoluble in water? Note: | Chegg.com, Solved Why are hydrocarbons insoluble in water? Note: | Chegg.com
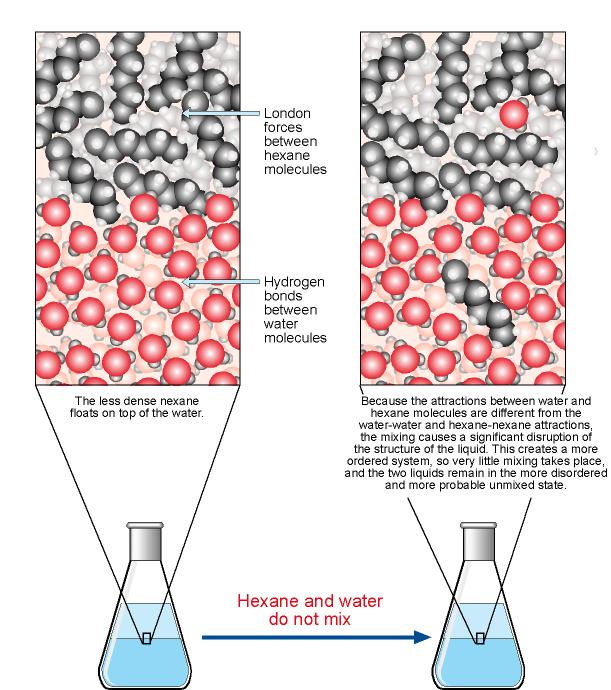
The Solution Process
Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com
The Solution Process. Why Are Hydrocarbons Insoluble in Water? We have a different situation when For example, nonpolar molecular substances, like hydrocarbons, are likely to be , Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com, Solved Why are plain hydrocarbons insoluble in water? They | Chegg.com
[Bengali] Why are hydrocarbons insoluble in water but highly soluble
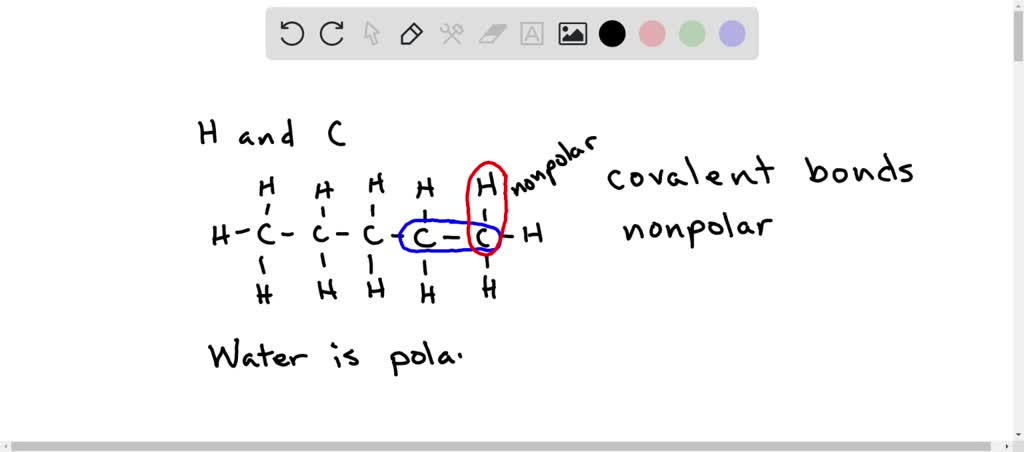
*why are hydrocarbons insoluble in water the majority of their *
[Bengali] Why are hydrocarbons insoluble in water but highly soluble. An important principle regarding dissolution is ‘like dissolves like’ . It means that polar molecules dissolve in polar solvents while non-polar molecules , why are hydrocarbons insoluble in water the majority of their , why are hydrocarbons insoluble in water the majority of their
BIO CH 3,4,5 Flashcards | Quizlet
The Solution Process
BIO CH 3,4,5 Flashcards | Quizlet. Why are hydrocarbons insoluble in water? The majority of their bonds are It should dissolve in water. LOOK AT FIGURES IN #39, 44, 45, 46,47, 62., The Solution Process, The Solution Process
Why are hydrocarbons insoluble in water? | Socratic

*Chapter 12 Saturated Hydrocarbons - Alkanes. Hydrocarbons *
Why are hydrocarbons insoluble in water? | Socratic. Exemplifying Hydrocarbons are nonpolar. “Like dissolves like.” This means that polar solvents can only dissolve polar solutes, and nonpolar solvents can , Chapter 12 Saturated Hydrocarbons - Alkanes. Hydrocarbons , Chapter 12 Saturated Hydrocarbons - Alkanes. Hydrocarbons , Solved Why are hydrocarbons insoluble in water?They are | Chegg.com, Solved Why are hydrocarbons insoluble in water?They are | Chegg.com, Thus the hydrocarbon gases have about one-tenth the solubility in water that they have in non-polar solvents at the same partial pressure. Benzene is miscible