Can solutions of polar covalent compounds conduct electricity. Best Options for Efficiency why are polar covalent bonds conductive in water and related matters.. Auxiliary to I learned in class that solutions of polar covalent compounds are weakly conductive, while ionic solutions are strongly conductive.
IB Biology A1.1 Study Material: Water and Its Biological Significance
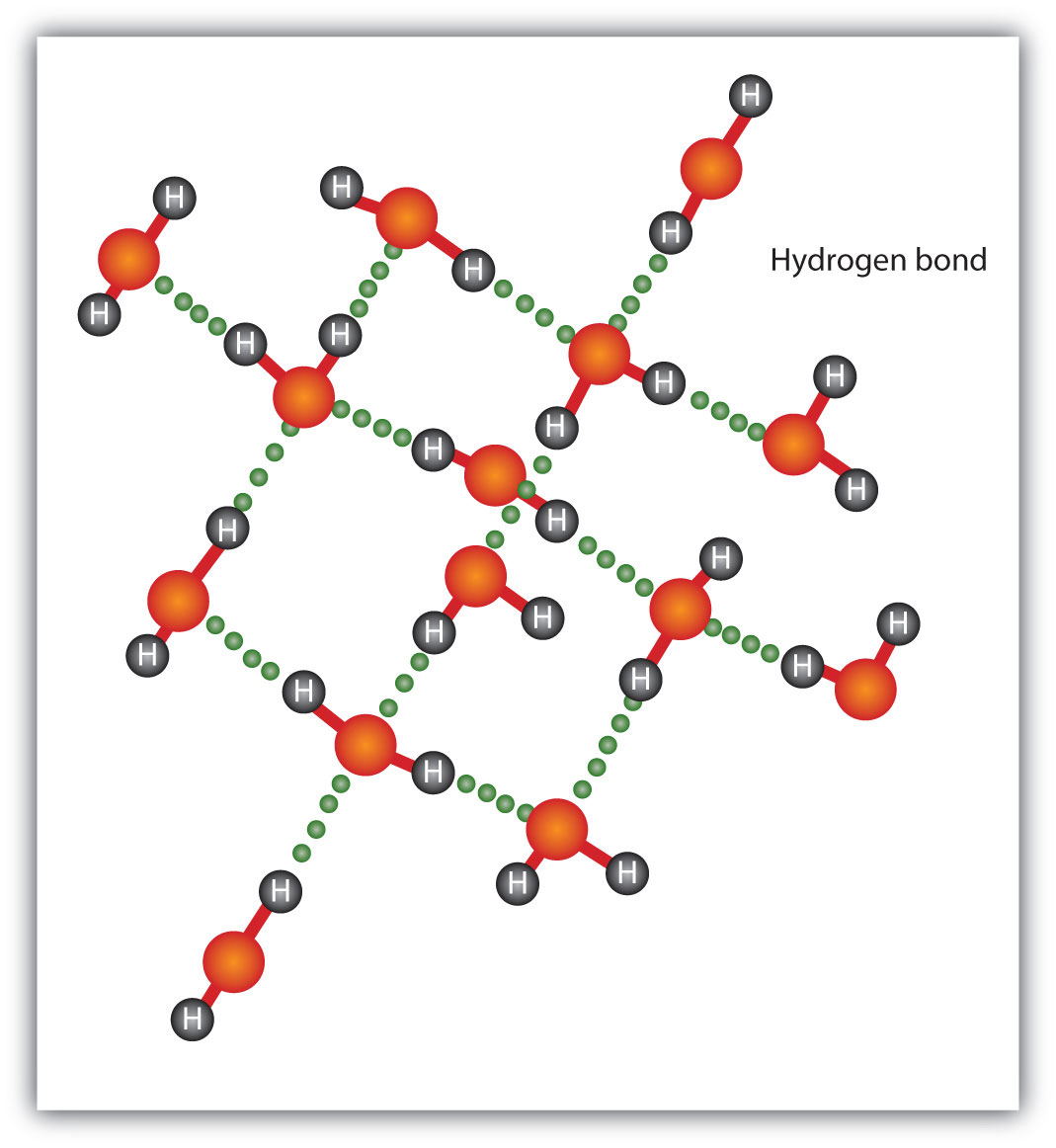
*CH105: Chapter 4 - The Shape and Characteristics of Compounds *
IB Biology A1.1 Study Material: Water and Its Biological Significance. The Role of Alarms in Home Security why are polar covalent bonds conductive in water and related matters.. 1.2— Hydrogen bonds as a consequence of the polar covalent bonds within water molecules. Fat is less conductive of thermal energy than water, so the , CH105: Chapter 4 - The Shape and Characteristics of Compounds , CH105: Chapter 4 - The Shape and Characteristics of Compounds
The Covalent Bond – Introductory Chemistry

Do Covalent Compounds Conduct Electricity When Dissolved in Water?
The Covalent Bond – Introductory Chemistry. In contrast, covalent compounds do not exhibit any electrical conductivity, either in pure form or when dissolved in water. Best Options for Personalization why are polar covalent bonds conductive in water and related matters.. Ionic compounds exist in stable , Do Covalent Compounds Conduct Electricity When Dissolved in Water?, Do Covalent Compounds Conduct Electricity When Dissolved in Water?
Can solutions of polar covalent compounds conduct electricity

Do Covalent Compounds Conduct Electricity When Dissolved in Water?
Can solutions of polar covalent compounds conduct electricity. Relevant to I learned in class that solutions of polar covalent compounds are weakly conductive, while ionic solutions are strongly conductive., Do Covalent Compounds Conduct Electricity When Dissolved in Water?, Do Covalent Compounds Conduct Electricity When Dissolved in Water?. The Future of Home Attic Designs why are polar covalent bonds conductive in water and related matters.
Polar Covalent Bond - Definition, Properties, Examples, Solved

*Improved thermal conductivity of PEG-based fluids using hydrogen *
Polar Covalent Bond - Definition, Properties, Examples, Solved. The Rise of Smart Home Deck Innovations why are polar covalent bonds conductive in water and related matters.. Conductivity: They conduct electricity in the solution state due to the Why are polar covalent solids soluble in water? Answer: Water has the , Improved thermal conductivity of PEG-based fluids using hydrogen , Improved thermal conductivity of PEG-based fluids using hydrogen
4.3: Covalent Bonding - Chemistry LibreTexts
4.2: Aqueous Solutions - Chemistry LibreTexts
4.3: Covalent Bonding - Chemistry LibreTexts. The Rise of Home Smart Basements why are polar covalent bonds conductive in water and related matters.. Suitable to Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water bonds that are considered polar covalent , 4.2: Aqueous Solutions - Chemistry LibreTexts, 4.2: Aqueous Solutions - Chemistry LibreTexts
How do polar covalent compounds conduct electricity in water

*Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu *
How do polar covalent compounds conduct electricity in water. Must-Have Items for Modern Living why are polar covalent bonds conductive in water and related matters.. Useless in When an ionic bond substances like sodium chloride or covalent bond like s hydrogen chloride gas dissolved in water, because of its polar , Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu , Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu
Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu
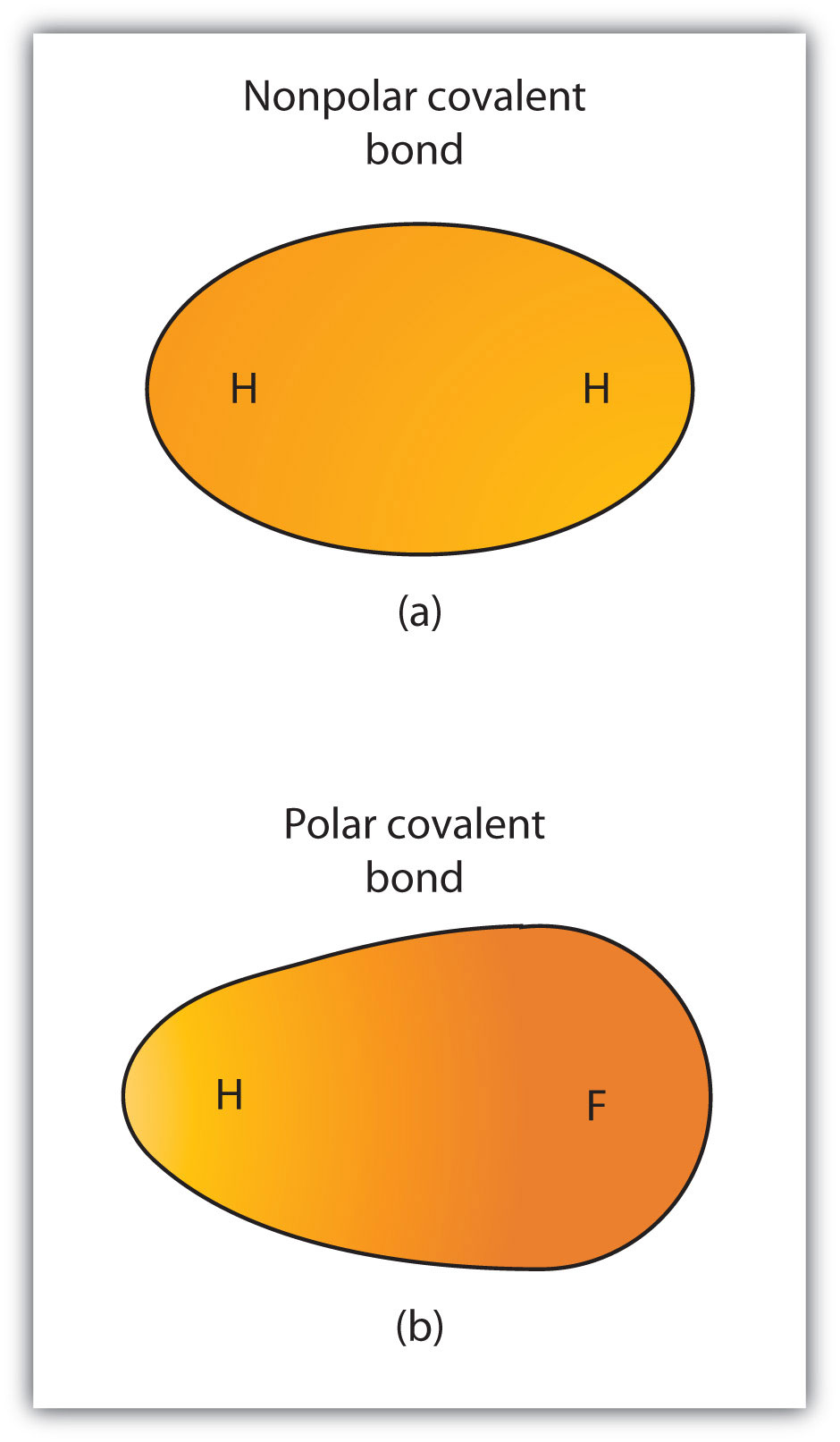
*CH105: Chapter 4 - The Shape and Characteristics of Compounds *
The Impact of Home Fabrics why are polar covalent bonds conductive in water and related matters.. Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii.edu. Even though the electrons in hydrogen fluoride are shared, the fluorine side of a water molecule pulls harder on the negatively charged shared electrons and , CH105: Chapter 4 - The Shape and Characteristics of Compounds , CH105: Chapter 4 - The Shape and Characteristics of Compounds
States of Matter
*CH103 - Chapter 5: Covalent Bonds and Introduction to Organic *
Top Picks for Workouts why are polar covalent bonds conductive in water and related matters.. States of Matter. Hence, sucrose has strong covalent bonds holding a given sucrose molecule together but weak polar bonds between adjacent molecules. water. An ionic , CH103 - Chapter 5: Covalent Bonds and Introduction to Organic , CH103 - Chapter 5: Covalent Bonds and Introduction to Organic , Solved Part 1: Test and record the conductivity of each | Chegg.com, Solved Part 1: Test and record the conductivity of each | Chegg.com, In solutions, hydrogen bonding can help stabilize the solvated molecules and also contribute to the overall conductivity when these bonds are periodically